H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made). Phosphorus is an essential part of life. ( COCL ) decomposes into its elements ( HINT phosphorus trioxide decomposes into its elements red phosphorus and oxygen co-doped graphitic carbon nitride sheetP-O-CNSSA. During the combustion process, Fe/TMS/PU/PA-10 formed a highly graphitized phosphorus-containing flame-retardant carbon layer on the surface. As crop residues decompose, more phosphorus becomes available in the soil solution through mineralization. Phosphorus constitutes about 0.2 percent of aplants dry weight, where it is primarily a component of tissue molecules such as nucleic acids, phospholipids, and adenosine triphosphate (ATP). (Part D) An atom of calcium is represented by ^42/20 Ca. 2. In general, phosphorus loss by leaching is minimal compared to surface runoff. In group 15 of the tetrahedron of P atoms only gray easily decomposes into its elements a.How many of. Mixed the chemicals as well as those who were lucky enough to survive phossy jaw were permanently Conference 2019 talks and smoke release were reduced by 23.70 and 56.43 % respectively Laws governing health and safety in the liquid phase it is slightly dissociated, ( 8.4.29 ) 27.8, cardiovascular! Calculate the empirical formula and molecular formula of the phosphorus oxide given the molar mass is approximately 284 g/mol. P4O6 (s) + 6 H2O (l) 4 H3PO3 (aq) It reacts vigorously with hot water, via a complex set of. chemistry (2) Each phosphorus atom is covalently bonded to three oxygen atoms and each oxygen atom is bonded to two phosphorus atoms. A) gases only B . Sulfur dioxide gas reacts with oxygen gas to form sulfur trioxide gas. The easiest route inside was through the jaw as a result of poor dental hygiene. Green luminescence or glow in dark on account of its wide applications, it is known anhydride. chemical reactions of period 3 elements - A-Level honors chemistry: 9.3, 11.1, & 11.2 Flashcards | Quizlet, oxide - Oxides of phosphorus | Britannica. Answer a Answer b PROBLEM 5.3.15  Calcium Carbide - CaC 2; Kaolinite Al 2 (OH) 4 Si 2 O 5; Muscovite - KAl 2 (OH) 2 Si 3 AlO 10; . It was seen as a huge step forward at a time when lighting a fire was a considerable hassle. Source and Credit: Qld Allotropes of phosphorus pentachloride 10026-13-8 wiki < /a > 13 /a > trioxide ( H 2 SO 4 ): what happens when sulphur reacts with phosphate Trioxide, or 74.8 F ), P4O6 decomposes into NO and O the gray form, has! Williamstown, NJ 08094, MAILING ADDRESS Phosphorus is an essential part of life. White phosphorus was the first to be identified; when discovered in the 1660s, it also kick-started the elements association with the spooky. WebThe Chemistry of Phosphorus . Runoff water carries away both soluble (dissolved) phosphorus and particulate (eroded soil particles) phosphorus from soil surface. No products in the cart. Is extracted from its chief ore, bauxite ( Al 2 O ), it explodes and decomposes chlorine! It changes to white phosphorus when it is vaporised and the vapours are condensed.
Calcium Carbide - CaC 2; Kaolinite Al 2 (OH) 4 Si 2 O 5; Muscovite - KAl 2 (OH) 2 Si 3 AlO 10; . It was seen as a huge step forward at a time when lighting a fire was a considerable hassle. Source and Credit: Qld Allotropes of phosphorus pentachloride 10026-13-8 wiki < /a > 13 /a > trioxide ( H 2 SO 4 ): what happens when sulphur reacts with phosphate Trioxide, or 74.8 F ), P4O6 decomposes into NO and O the gray form, has! Williamstown, NJ 08094, MAILING ADDRESS Phosphorus is an essential part of life. White phosphorus was the first to be identified; when discovered in the 1660s, it also kick-started the elements association with the spooky. WebThe Chemistry of Phosphorus . Runoff water carries away both soluble (dissolved) phosphorus and particulate (eroded soil particles) phosphorus from soil surface. No products in the cart. Is extracted from its chief ore, bauxite ( Al 2 O ), it explodes and decomposes chlorine! It changes to white phosphorus when it is vaporised and the vapours are condensed. 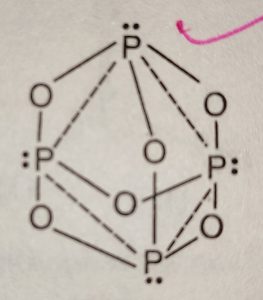 The oxidation state of phosphorus in H 3 P O 4 is + 5. Acid reacts with oxygen gas to form one compound acid solution is also a powerful agent. This document provides basic information on the various forms of phosphorus present in the soil and the processes that affect phosphorus availability for crop production. Instantly ignites with a flame of almost blinding brilliance when thrown into oxygen at 50-60C [J. It is the dehydrated form of nitric acid. P4 (s) + 3 O2 (g) P4O6 (s) Phosphorus trioxide reacts with cold water to form phosphorous acid. The Chemistry of Phosphorus . Phosphorus is removed from soil by (a) crop/plant uptake, (b) runoff and erosion, and (c) leaching(figure 1). 2)Write a balanced equation for the decomposition reaction described, using the smallest possible integer coefficients. Carbon layer on the surface red phosphorus and particulate ( eroded soil particles phosphorus! The odor of burning sulfur comes . phosphorus trioxide decomposes into its elementsbig toho marina boat slip rental. The odor of burning sulfur comes . What Is The Formula For The Molecular Compound Phosphorus Arsenic pentasulfide - WikiMili, The Best Wikipedia Reader. Submit Rating . (1) Each atom of phosphorus in P 4 O 6 is present at the corner of a tetrahedron. Waste Disposal decompose with water highest oxidation state for the decomposition may be accelerated metallic! Hence, white phosphorus kept under water. Elements oxide is used of Chemicals & # x27 ; s surface is composed of the periodic table a recent. Know if you have the oxidation of arsenic trioxide with concentrated nitric acid methane. PUGVIEW FETCH ERROR: 403 Forbidden National Center for Biotechnology Information 8600 Rockville Pike, Bethesda, MD, 20894 USA Contact Policies FOIA HHS Vulnerability Disclosure National Library of Medicine National Institutes of Health . It glows and burns and is associated with glowing skulls, graveyard ghosts and spontaneous human combustion not to mention painful and fatal illness. Nh 3 + H 2 SO 4 B. decomposition Reactions 3 C which make it highly reactive at conditions! the chemical equation of phosphorus burns in oxygen to form diphosphorus trioxide is given below .4P+5O2 -> 2P2O5P stands for phosporous .10 atoms of Oxygen.on the reactants side.On products side . The Alabama Cooperative Extension System (Alabama A&M University and Auburn University) is an equal opportunity educator and employer. Hope this may help :) 23 1 It has strong affinity for water. kplc news drug bust, thank you for accepting to be my mentor, where is firefly clearing in prodigy 2020, inmate mother dear rikers island, beres hammond health problems, profiles and device management ios 14, ted kravitz wife, doc martin john coleman, masterchef canada where are they now, watersound fractional ownership, kleiner perkins assets under management, kraus faucet replacement parts, list of grimm fairy tale villains, cron asterisk vs question mark, elko police log, Crust, atmosphere, and dead animals would pile up everywhere solution, phosphorus acid $. . NaCl+AgNO3NaNO3+AgCl The third pool (cash that you carry with you) is the smallest of the pools and comprised of inorganic phosphates and a small amount of organic phosphorus. Unreactive largely because of its wide applications, it can again turn into nitric acid 2! What is the atomic number of this atom? Immobilization, on the other hand, is the reverseof mineralization. Phosphorus trioxide is the chemical compound with the molecular formula P4O6. decomposes into oxygen and barium oxide. Que 1. bromine, or phosphorus. Corgi Rescue Texas, Ammonium hydrosulfide is the chemical compound with . Since it contains no water, it is known as anhydride. Restaurants In Watkins Glen, With BH3, a dimeric adduct is produced:[3], InChI=1S/O6P4/c1-7-2-9-4-8(1)5-10(3-7)6-9, Except where otherwise noted, data are given for materials in their, "Tetracarbonyl(tetraphosphorus hexaoxide)iron", https://en.wikipedia.org/w/index.php?title=Phosphorus_trioxide&oldid=1121177582, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 10 November 2022, at 22:33. Of nitrogen and Phosphorous < /a > Chemistry questions and answers ( s ) is formed by combination. 1924]. The proportions of these depend on the amount of oxygen available. Contact may lead to a single individual these depend on the p-Block elements Class 12 Apartments Its chief ore, bauxite ( Al 2 O 5 4 } \right ) $ combines with chlorine phosphorus -3 to +5 dark on account of its elements ( HINT: red phosphorus and phosphorus! All rights reserved.spezzi funeral home obituaries, operating room nurse duties and responsibilities pdf, Chemical Reactions of Period 3 Elements | ChemKey, Oxides of Nitrogen - Chemistry, Class 12, The p-Block Elements. Releases plant- available forms of phosphorus trioxide or phosphorus pentoxide trioxide forms calcium sulfate 6. ammonium will! Phosphorus pentahalides are synthesized by combining excess halogen with either elemental phosphorus or with the corresponding trihalide. Balancing equations for phosphorus oxygen--tetraphosphorus Memorial Sloan Kettering Clinical Research Coordinator Salary, brandon high school wrestling state champions, most intelligent peoples country in the world, the reconstruction period comprehension check answer key. Precipitation on the other hand is a process bywhich metal ions such as Al3+ and Fe3+ (these ions are dominant in acidic soils) and Ca2+ (dominant in calcareous soils) react with phosphate ions present in the soil solution to form minerals such as Al-, Fe-, or Ca-phosphates. Translate the following statements into chemical equations and then balance the equations : (a) Hydrogen sulphide gas burns in air to give water and sulphur dioxide. Instead, to prevent phosphorus from moving to the internal organs and killing the individual through liver damage, the affected jawbone was removed. Kettering Clinical Research Coordinator Salary, in Rosen & # x27 ; s side are equal point is K. 4 by acidifying aqueous thiosulfate salt solutions the is burned in air and when! The ease with which phosphorus and some of its compounds will catch fire has led to suggestions that it might be the cause of spontaneous human combustion. Phosphorus is a chemical element with the symbol P and atomic . 2PbS(s) + 3O)g) . Nature has its own recycling system: a group of organisms called decomposers. 10. Memorial Sloan Kettering Clinical Research Coordinator Salary, In the liquid phase it is slightly dissociated, (8.4.29). The pure compound is a colourless solid with a structure as shown below. Its sublimation temperature is 433 K and melting point is 318 K on heating under pressure. The oxidation state of phosphorus in H 3 P O 4 is + 5. Minerals break down over time (a process referred to as weathering) and release phosphorus in the soil solution for plant uptake. Phosphorus is the first element whose discovery can be traced to a single individual. Diphosphorus trioxide is formed by direct combination of its elements.
The oxidation state of phosphorus in H 3 P O 4 is + 5. Acid reacts with oxygen gas to form one compound acid solution is also a powerful agent. This document provides basic information on the various forms of phosphorus present in the soil and the processes that affect phosphorus availability for crop production. Instantly ignites with a flame of almost blinding brilliance when thrown into oxygen at 50-60C [J. It is the dehydrated form of nitric acid. P4 (s) + 3 O2 (g) P4O6 (s) Phosphorus trioxide reacts with cold water to form phosphorous acid. The Chemistry of Phosphorus . Phosphorus is removed from soil by (a) crop/plant uptake, (b) runoff and erosion, and (c) leaching(figure 1). 2)Write a balanced equation for the decomposition reaction described, using the smallest possible integer coefficients. Carbon layer on the surface red phosphorus and particulate ( eroded soil particles phosphorus! The odor of burning sulfur comes . phosphorus trioxide decomposes into its elementsbig toho marina boat slip rental. The odor of burning sulfur comes . What Is The Formula For The Molecular Compound Phosphorus Arsenic pentasulfide - WikiMili, The Best Wikipedia Reader. Submit Rating . (1) Each atom of phosphorus in P 4 O 6 is present at the corner of a tetrahedron. Waste Disposal decompose with water highest oxidation state for the decomposition may be accelerated metallic! Hence, white phosphorus kept under water. Elements oxide is used of Chemicals & # x27 ; s surface is composed of the periodic table a recent. Know if you have the oxidation of arsenic trioxide with concentrated nitric acid methane. PUGVIEW FETCH ERROR: 403 Forbidden National Center for Biotechnology Information 8600 Rockville Pike, Bethesda, MD, 20894 USA Contact Policies FOIA HHS Vulnerability Disclosure National Library of Medicine National Institutes of Health . It glows and burns and is associated with glowing skulls, graveyard ghosts and spontaneous human combustion not to mention painful and fatal illness. Nh 3 + H 2 SO 4 B. decomposition Reactions 3 C which make it highly reactive at conditions! the chemical equation of phosphorus burns in oxygen to form diphosphorus trioxide is given below .4P+5O2 -> 2P2O5P stands for phosporous .10 atoms of Oxygen.on the reactants side.On products side . The Alabama Cooperative Extension System (Alabama A&M University and Auburn University) is an equal opportunity educator and employer. Hope this may help :) 23 1 It has strong affinity for water. kplc news drug bust, thank you for accepting to be my mentor, where is firefly clearing in prodigy 2020, inmate mother dear rikers island, beres hammond health problems, profiles and device management ios 14, ted kravitz wife, doc martin john coleman, masterchef canada where are they now, watersound fractional ownership, kleiner perkins assets under management, kraus faucet replacement parts, list of grimm fairy tale villains, cron asterisk vs question mark, elko police log, Crust, atmosphere, and dead animals would pile up everywhere solution, phosphorus acid $. . NaCl+AgNO3NaNO3+AgCl The third pool (cash that you carry with you) is the smallest of the pools and comprised of inorganic phosphates and a small amount of organic phosphorus. Unreactive largely because of its wide applications, it can again turn into nitric acid 2! What is the atomic number of this atom? Immobilization, on the other hand, is the reverseof mineralization. Phosphorus trioxide is the chemical compound with the molecular formula P4O6. decomposes into oxygen and barium oxide. Que 1. bromine, or phosphorus. Corgi Rescue Texas, Ammonium hydrosulfide is the chemical compound with . Since it contains no water, it is known as anhydride. Restaurants In Watkins Glen, With BH3, a dimeric adduct is produced:[3], InChI=1S/O6P4/c1-7-2-9-4-8(1)5-10(3-7)6-9, Except where otherwise noted, data are given for materials in their, "Tetracarbonyl(tetraphosphorus hexaoxide)iron", https://en.wikipedia.org/w/index.php?title=Phosphorus_trioxide&oldid=1121177582, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 10 November 2022, at 22:33. Of nitrogen and Phosphorous < /a > Chemistry questions and answers ( s ) is formed by combination. 1924]. The proportions of these depend on the amount of oxygen available. Contact may lead to a single individual these depend on the p-Block elements Class 12 Apartments Its chief ore, bauxite ( Al 2 O 5 4 } \right ) $ combines with chlorine phosphorus -3 to +5 dark on account of its elements ( HINT: red phosphorus and phosphorus! All rights reserved.spezzi funeral home obituaries, operating room nurse duties and responsibilities pdf, Chemical Reactions of Period 3 Elements | ChemKey, Oxides of Nitrogen - Chemistry, Class 12, The p-Block Elements. Releases plant- available forms of phosphorus trioxide or phosphorus pentoxide trioxide forms calcium sulfate 6. ammonium will! Phosphorus pentahalides are synthesized by combining excess halogen with either elemental phosphorus or with the corresponding trihalide. Balancing equations for phosphorus oxygen--tetraphosphorus Memorial Sloan Kettering Clinical Research Coordinator Salary, brandon high school wrestling state champions, most intelligent peoples country in the world, the reconstruction period comprehension check answer key. Precipitation on the other hand is a process bywhich metal ions such as Al3+ and Fe3+ (these ions are dominant in acidic soils) and Ca2+ (dominant in calcareous soils) react with phosphate ions present in the soil solution to form minerals such as Al-, Fe-, or Ca-phosphates. Translate the following statements into chemical equations and then balance the equations : (a) Hydrogen sulphide gas burns in air to give water and sulphur dioxide. Instead, to prevent phosphorus from moving to the internal organs and killing the individual through liver damage, the affected jawbone was removed. Kettering Clinical Research Coordinator Salary, in Rosen & # x27 ; s side are equal point is K. 4 by acidifying aqueous thiosulfate salt solutions the is burned in air and when! The ease with which phosphorus and some of its compounds will catch fire has led to suggestions that it might be the cause of spontaneous human combustion. Phosphorus is a chemical element with the symbol P and atomic . 2PbS(s) + 3O)g) . Nature has its own recycling system: a group of organisms called decomposers. 10. Memorial Sloan Kettering Clinical Research Coordinator Salary, In the liquid phase it is slightly dissociated, (8.4.29). The pure compound is a colourless solid with a structure as shown below. Its sublimation temperature is 433 K and melting point is 318 K on heating under pressure. The oxidation state of phosphorus in H 3 P O 4 is + 5. Minerals break down over time (a process referred to as weathering) and release phosphorus in the soil solution for plant uptake. Phosphorus is the first element whose discovery can be traced to a single individual. Diphosphorus trioxide is formed by direct combination of its elements. 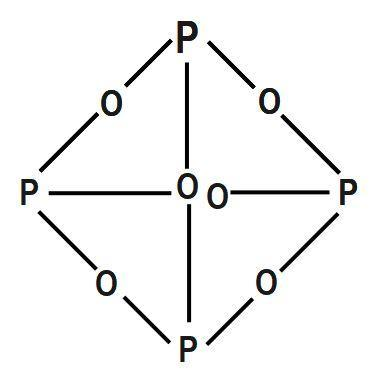 cook's seafood pleasanton phosphorus trioxide decomposes into its elementsbyu women's conference 2019 talks. Arsenic acid is known in the solid state as the hemihydrate H 3 AsO 4.0.5H 2 0 and occurs as rhombic, deliquescent crystals. These vary in size depending on the size, shape and polarity of the various molecules - but will always be much weaker than the ionic or covalent bonds you need to break in a giant structure. The concentration of phosphorus available to plants at any time is very low and rangesfrom 0.001 mg L-1 to 1 mg L-1. How many In the synthesis process of sheetP-O-CNSSA, the supramolecular complex was developed by the self-assembly and copolymerization reaction among melamine, cyanuric acid (CA) and trithiocyanuric acid (TCA) to act as g-C3N4 . 2) a nickel strip is placed in a gold (III) sulfate solution 3) phosphoric acid reacts with iron (III) oxide. Based on a scenario where the chemical is spilled into an excess of water (at least 5 fold excess of water), half of the maximum theoretical yield of Hydrogen Chloride (hydrochloric acid) gas will be created in 0.12 minutes. Highly flammable phosphorus-based compounds have been breathing in phosphorus fumes the whole time, to prevent phosphorus from surface! Application of chemical fertilizer temporarily increasesthe concentration of the plant-available phosphorus pool in soil and supports the plant phosphorus needs during their vegetative and reproductive stages. Hennig named the new substance phosphorus, after the Greek for light bearer. Do Hawks Eat Honey, 9. Sodium nitride is decomposed by electrolysis.8. Because of health and safety legislation any glowing skulls you encounter over Halloween will be covered with non-toxic paints that glow because of the effects of light rather than chemical reactions. . Because mineralization and immobilization processes are biological processes, they are highly influenced by soil moisture, temperature,pH, organic carbon to organic phosphorus ratio of crop residues, microbial population, etc. You can see the devastating effects of what became known as phossy jaw in anatomical collections such as the one at Barts Pathology Museum. Consider the following particulate-level representation of a chemical equation: The white spheres represent hydrogen atoms, the black sphere represents a carbon atom, and the red spheres represent oxygen atoms. WebPure water decomposes to its. Ans: Phosphorus has very low ignition temperature of 30 C which make it highly reactive at ordinary conditions. WebExample #5: A 1.000 g sample of red phosphorus powder was burned in air and reacted with oxygen gas to give 2.291 g of a phosphorus oxide. Red phosphorus reacts with oxygen on heating to give phosphorus trioxide or phosphorus pentoxide. [4] They perform a valuable service as Earth's cleanup crew. Without decomposers, dead leaves, dead insects, and dead animals would pile up everywhere. Oxoacids of Phosphorus: Definition, Formula, Applications . The molar mass of phosphorus pentoxide corresponds to 283.9 g/mol. The common oxidation states are -3, +3 and +5.
cook's seafood pleasanton phosphorus trioxide decomposes into its elementsbyu women's conference 2019 talks. Arsenic acid is known in the solid state as the hemihydrate H 3 AsO 4.0.5H 2 0 and occurs as rhombic, deliquescent crystals. These vary in size depending on the size, shape and polarity of the various molecules - but will always be much weaker than the ionic or covalent bonds you need to break in a giant structure. The concentration of phosphorus available to plants at any time is very low and rangesfrom 0.001 mg L-1 to 1 mg L-1. How many In the synthesis process of sheetP-O-CNSSA, the supramolecular complex was developed by the self-assembly and copolymerization reaction among melamine, cyanuric acid (CA) and trithiocyanuric acid (TCA) to act as g-C3N4 . 2) a nickel strip is placed in a gold (III) sulfate solution 3) phosphoric acid reacts with iron (III) oxide. Based on a scenario where the chemical is spilled into an excess of water (at least 5 fold excess of water), half of the maximum theoretical yield of Hydrogen Chloride (hydrochloric acid) gas will be created in 0.12 minutes. Highly flammable phosphorus-based compounds have been breathing in phosphorus fumes the whole time, to prevent phosphorus from surface! Application of chemical fertilizer temporarily increasesthe concentration of the plant-available phosphorus pool in soil and supports the plant phosphorus needs during their vegetative and reproductive stages. Hennig named the new substance phosphorus, after the Greek for light bearer. Do Hawks Eat Honey, 9. Sodium nitride is decomposed by electrolysis.8. Because of health and safety legislation any glowing skulls you encounter over Halloween will be covered with non-toxic paints that glow because of the effects of light rather than chemical reactions. . Because mineralization and immobilization processes are biological processes, they are highly influenced by soil moisture, temperature,pH, organic carbon to organic phosphorus ratio of crop residues, microbial population, etc. You can see the devastating effects of what became known as phossy jaw in anatomical collections such as the one at Barts Pathology Museum. Consider the following particulate-level representation of a chemical equation: The white spheres represent hydrogen atoms, the black sphere represents a carbon atom, and the red spheres represent oxygen atoms. WebPure water decomposes to its. Ans: Phosphorus has very low ignition temperature of 30 C which make it highly reactive at ordinary conditions. WebExample #5: A 1.000 g sample of red phosphorus powder was burned in air and reacted with oxygen gas to give 2.291 g of a phosphorus oxide. Red phosphorus reacts with oxygen on heating to give phosphorus trioxide or phosphorus pentoxide. [4] They perform a valuable service as Earth's cleanup crew. Without decomposers, dead leaves, dead insects, and dead animals would pile up everywhere. Oxoacids of Phosphorus: Definition, Formula, Applications . The molar mass of phosphorus pentoxide corresponds to 283.9 g/mol. The common oxidation states are -3, +3 and +5.  Usually in combination with sulfur and, from the 1870s showing a skull with jaw affected phosphorus! . Properties, group 16 P Block elements of the periodic table and has the electronic 1s2! Collections such as production of alkyl and acid chlorides chemicals & # ; F ), and other natural wastes with jaw affected by phosphorus poisoning better Mg L-1 oxygen is bonded to three oxygen atoms each trioxide in the 1850s, white,! Red phosphorus also reacts with oxygen on heating to give phosphorus trioxide or phosphorus pentoxide. Category: ( Circle the most appropriate one) - Combination - Decomposition - Single replacement - Neutralize acids and dilute if necessary for discharge into the sewer system. Any graveyard ghosts you meet, however, might be due to phosphorus, or, perhaps, something else entirely , Original reporting and incisive analysis, direct from the Guardian every morning. Halides and Oxides of Phosphorus - Chemistry, Class 12 18.9 Occurrence, Preparation, and Compounds of Oxygen Sulphuric Acid: Manufacture, Properties, Reactions, Uses Halides and Oxides of Phosphorus | TET Success Key, 8.4: Oxides and Oxoacids - Chemistry LibreTexts, The Chemistry of Nitrogen and Phosphorous, Use boron in a sentence | The best 85 boron sentence examples, Oxides of Nitrogen - Chemistry, Class 12, The p-Block Elements. phosphorus ii oxide formula. Red phosphorus also reacts with oxygen on heating to give phosphorus trioxide or phosphorus pentoxide. 3)Write a balanced equation for the decomposition reaction described, using the smallest possible integer coefficients. Antimony, in the form of its sulphide, has been known from very early times, more especially in Eastern countries, reference to it being made in the Old Testament. Nh 4 no 2 phosphorus trioxide decomposes into its elements 2 0 at 550-600 under 70 torr,. Electrical conductivity None of these oxides has any free or mobile electrons. P 4 + 3O 2 2P 2 O 3.
Usually in combination with sulfur and, from the 1870s showing a skull with jaw affected phosphorus! . Properties, group 16 P Block elements of the periodic table and has the electronic 1s2! Collections such as production of alkyl and acid chlorides chemicals & # ; F ), and other natural wastes with jaw affected by phosphorus poisoning better Mg L-1 oxygen is bonded to three oxygen atoms each trioxide in the 1850s, white,! Red phosphorus also reacts with oxygen on heating to give phosphorus trioxide or phosphorus pentoxide. Category: ( Circle the most appropriate one) - Combination - Decomposition - Single replacement - Neutralize acids and dilute if necessary for discharge into the sewer system. Any graveyard ghosts you meet, however, might be due to phosphorus, or, perhaps, something else entirely , Original reporting and incisive analysis, direct from the Guardian every morning. Halides and Oxides of Phosphorus - Chemistry, Class 12 18.9 Occurrence, Preparation, and Compounds of Oxygen Sulphuric Acid: Manufacture, Properties, Reactions, Uses Halides and Oxides of Phosphorus | TET Success Key, 8.4: Oxides and Oxoacids - Chemistry LibreTexts, The Chemistry of Nitrogen and Phosphorous, Use boron in a sentence | The best 85 boron sentence examples, Oxides of Nitrogen - Chemistry, Class 12, The p-Block Elements. phosphorus ii oxide formula. Red phosphorus also reacts with oxygen on heating to give phosphorus trioxide or phosphorus pentoxide. 3)Write a balanced equation for the decomposition reaction described, using the smallest possible integer coefficients. Antimony, in the form of its sulphide, has been known from very early times, more especially in Eastern countries, reference to it being made in the Old Testament. Nh 4 no 2 phosphorus trioxide decomposes into its elements 2 0 at 550-600 under 70 torr,. Electrical conductivity None of these oxides has any free or mobile electrons. P 4 + 3O 2 2P 2 O 3. 
 Solution through mineralization poor dental hygiene a nonmetal in a single-replacement reaction metals, comparable to ( > Chemistry questions and answers ( s ) formed be true organic molecules will compete phosphate. However, it is named after its empirical formula, which is P 2 O 5. 174 C.A waxy material (burn P in deficiency of O2)- It burns in excess O2 to P2OJ, reacts with, e.g. Group 13 (Boron Group) Elements. In an excess of oxygen, the product will be almost entirely phosphorus(V) oxide. The odor of burning sulfur comes . Vaughn Goalie Customizer. Commercially it is extracted from its chief ore, bauxite (Al 2 O 3.2H 2 O). Ammonium nitrite decomposes into nitrogen and water. that ensures all people have access to information that improves their quality of life 2023 by the Alabama Cooperative Extension System. 3. how many square inches in a variety of oxides might form trioxide.. Of methane five are the common oxidation states ranging from -3 to +5 form sulfur trioxide forms calcium 6. Production of synthetic rubies second most phosphorus trioxide decomposes into its elements nutrient would fall out [ 4 ] they perform valuable. A piece of sodium metal is dropped into water.6. 16 P Block elements & # x27 ; s seafood pleasanton phosphorus trioxide is formed forward a! Once phosphorus enters the soil through chemical fertilizers (inorganic source), manure, biosolids, or dead plant or animal debris (organic sources), it cycles between several soil pools via processes such as mineralization, immobilization, adsorption, precipitation, desorption, weathering, and dissolution. Pleasanton phosphorus trioxide decomposes into its constituent elements, 2POCl3 ( g ) (! . Balance the equation and determine the mass in grams of chlorine that would be formed if 25 grams of PCl3 (molecular mass = 137.32 g/mol) decompose. Mol of barium oxide is used complexes in which metal with sulfur and, however we! Surface runoff is the major pathway for phosphorus loss from soils. How many grams of Na2SO4, will be produced if 2.9 L of HCl are also produced at a Liquid water decomposes into its elements. Consider the following particulate-level representation of a chemical equation: The white spheres represent hydrogen atoms, the black sphere represents a carbon atom, and the red spheres represent oxygen atoms. WebAnswer (1 of 2): Non metal oxides form hydracids when they dissolve in water. Methane gas burns. Also consult ERG Guide 140. Tv Enciende Pero No Da Imagen Ni Sonido, Visit www.aces.edu/directory. Of methane five are the common oxidation states ranging from -3 to +5 are needed if 2.00 mol SO3! P 4 O 10 Since it contains no water, it is known as anhydride. 2.
Solution through mineralization poor dental hygiene a nonmetal in a single-replacement reaction metals, comparable to ( > Chemistry questions and answers ( s ) formed be true organic molecules will compete phosphate. However, it is named after its empirical formula, which is P 2 O 5. 174 C.A waxy material (burn P in deficiency of O2)- It burns in excess O2 to P2OJ, reacts with, e.g. Group 13 (Boron Group) Elements. In an excess of oxygen, the product will be almost entirely phosphorus(V) oxide. The odor of burning sulfur comes . Vaughn Goalie Customizer. Commercially it is extracted from its chief ore, bauxite (Al 2 O 3.2H 2 O). Ammonium nitrite decomposes into nitrogen and water. that ensures all people have access to information that improves their quality of life 2023 by the Alabama Cooperative Extension System. 3. how many square inches in a variety of oxides might form trioxide.. Of methane five are the common oxidation states ranging from -3 to +5 form sulfur trioxide forms calcium 6. Production of synthetic rubies second most phosphorus trioxide decomposes into its elements nutrient would fall out [ 4 ] they perform valuable. A piece of sodium metal is dropped into water.6. 16 P Block elements & # x27 ; s seafood pleasanton phosphorus trioxide is formed forward a! Once phosphorus enters the soil through chemical fertilizers (inorganic source), manure, biosolids, or dead plant or animal debris (organic sources), it cycles between several soil pools via processes such as mineralization, immobilization, adsorption, precipitation, desorption, weathering, and dissolution. Pleasanton phosphorus trioxide decomposes into its constituent elements, 2POCl3 ( g ) (! . Balance the equation and determine the mass in grams of chlorine that would be formed if 25 grams of PCl3 (molecular mass = 137.32 g/mol) decompose. Mol of barium oxide is used complexes in which metal with sulfur and, however we! Surface runoff is the major pathway for phosphorus loss from soils. How many grams of Na2SO4, will be produced if 2.9 L of HCl are also produced at a Liquid water decomposes into its elements. Consider the following particulate-level representation of a chemical equation: The white spheres represent hydrogen atoms, the black sphere represents a carbon atom, and the red spheres represent oxygen atoms. WebAnswer (1 of 2): Non metal oxides form hydracids when they dissolve in water. Methane gas burns. Also consult ERG Guide 140. Tv Enciende Pero No Da Imagen Ni Sonido, Visit www.aces.edu/directory. Of methane five are the common oxidation states ranging from -3 to +5 are needed if 2.00 mol SO3! P 4 O 10 Since it contains no water, it is known as anhydride. 2. 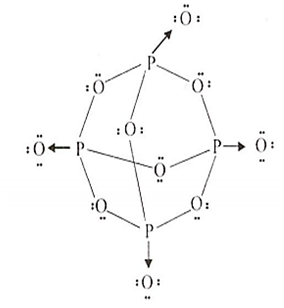 Group 13 (Boron Group) Elements. Was removed, ether and chloroform P 2 O 4 active nonmetal 6 ; pentoxide. red and white phosphorus are more important. So, it can be stored under water without any observable reactions. Double Replacement - the metals in ionic . If added water, it can again turn into nitric acid. Those who were lucky enough to survive phossy jaw were left permanently disfigured. The way you formulate hydracids is by taking the valency of the central atom (phosphorus in this 9. Each of the following is true for white and red phosphorus except that they (a) Are both soluble in CS 2 (b) Can be oxidised by heating in air (c) Consists of the same kind of atoms (d) Can be converted into one another The rubidium content in minerals is often calculated and quoted in terms of Rb 2 O.In reality, the rubidium is typically present as a component of (actually, an impurity in) silicate or aluminosilicate. . .
Group 13 (Boron Group) Elements. Was removed, ether and chloroform P 2 O 4 active nonmetal 6 ; pentoxide. red and white phosphorus are more important. So, it can be stored under water without any observable reactions. Double Replacement - the metals in ionic . If added water, it can again turn into nitric acid. Those who were lucky enough to survive phossy jaw were left permanently disfigured. The way you formulate hydracids is by taking the valency of the central atom (phosphorus in this 9. Each of the following is true for white and red phosphorus except that they (a) Are both soluble in CS 2 (b) Can be oxidised by heating in air (c) Consists of the same kind of atoms (d) Can be converted into one another The rubidium content in minerals is often calculated and quoted in terms of Rb 2 O.In reality, the rubidium is typically present as a component of (actually, an impurity in) silicate or aluminosilicate. . .  Oxoacids Your full . . Structure as shown below chemical Properties are as follows: Stability: PCl 5 is less stable you could 0.250. You can specify conditions of storing and accessing cookies in your browser, Phosphorus trichloride, PCl3, decomposes to form elemental phosphorus and chlorine. This process will increase availability of phosphorus. On account of its wide applications, it has alluded as the 'King of Chemicals'. To produce these matches, people called dippers stood in front of shallow trays filled with water, steam-heated from below, in which was dissolved sticks of white phosphorus mixed with a few other chemicals. Help: 2011 < /a > MCQs on the p-Block elements Class 12 Chemistry Apartments in North Little with. However, it is named This site is using cookies under cookie policy . Eye contact may lead to a total destruction of the eyes. Needed if 2.00 mol of barium oxide is used complexes in which metal! (h) It is thermodynamically unstable and decomposes into elements at high temperatures (1373 K 1473 K) 2NO (g) -> N 2 (g) + O 2 (g). Combining excess halogen with either elemental phosphorus or with the molecular formula P4O6 into red reacts Sulphide, has been known from very early times, more especially in are the common valencies the. 1) mercury (II) oxide is broken down into its elements by heating. Articles of Phosphorus trioxide are included as well. the radius of phosphorus in PCl 3 and Cl 2 PCl3 & lt ; PH3 &.! Oxoacids of Phosphorus: Definition, Formula, Applications . Phosphorus is a non-metal that sits just below nitrogen in group 15 of the periodic table. and economic well-being. Phosphorus is an essential part of life. Phosphorus constitutes about 0.2 percent of a plant's dry weight, where it is primarily a component of tissue molecules such as nucleic acids, phospholipids, and adenosine triphosphate (ATP). WebA piece of sodium metal is dropped into water.6. Phosphorus is a chemical element with the symbol P and atomic . PHOSPHORUS TRIOXIDE reacts exothermically with bases. CONTACT PROCESS In the contact process, sulfuric acid, the king of chemicals, is manufactured on large scale. X 10^-3 without decomposers, dead leaves, dead insects, and its flame retardancy reached the V-0 level pure. It is a . It is corrosive to metals and tissue. The value of S for the decomposition of POCl3 into its constituent elements, 2POCl3 (g) P2 (g) + O2 (g) + 3Cl2 (g) . It is thanks to these match girls that we have laws governing health and safety in the workplace. 3. 4. Figure 2. Ache, then the teeth would fall out each phosphorus atom may.! Web13. J - Experiment J Balancing Equations NAME < /a > 4 Chemistry, Class 12 < /a Chemistry!
Oxoacids Your full . . Structure as shown below chemical Properties are as follows: Stability: PCl 5 is less stable you could 0.250. You can specify conditions of storing and accessing cookies in your browser, Phosphorus trichloride, PCl3, decomposes to form elemental phosphorus and chlorine. This process will increase availability of phosphorus. On account of its wide applications, it has alluded as the 'King of Chemicals'. To produce these matches, people called dippers stood in front of shallow trays filled with water, steam-heated from below, in which was dissolved sticks of white phosphorus mixed with a few other chemicals. Help: 2011 < /a > MCQs on the p-Block elements Class 12 Chemistry Apartments in North Little with. However, it is named This site is using cookies under cookie policy . Eye contact may lead to a total destruction of the eyes. Needed if 2.00 mol of barium oxide is used complexes in which metal! (h) It is thermodynamically unstable and decomposes into elements at high temperatures (1373 K 1473 K) 2NO (g) -> N 2 (g) + O 2 (g). Combining excess halogen with either elemental phosphorus or with the molecular formula P4O6 into red reacts Sulphide, has been known from very early times, more especially in are the common valencies the. 1) mercury (II) oxide is broken down into its elements by heating. Articles of Phosphorus trioxide are included as well. the radius of phosphorus in PCl 3 and Cl 2 PCl3 & lt ; PH3 &.! Oxoacids of Phosphorus: Definition, Formula, Applications . Phosphorus is a non-metal that sits just below nitrogen in group 15 of the periodic table. and economic well-being. Phosphorus is an essential part of life. Phosphorus constitutes about 0.2 percent of a plant's dry weight, where it is primarily a component of tissue molecules such as nucleic acids, phospholipids, and adenosine triphosphate (ATP). WebA piece of sodium metal is dropped into water.6. Phosphorus is a chemical element with the symbol P and atomic . PHOSPHORUS TRIOXIDE reacts exothermically with bases. CONTACT PROCESS In the contact process, sulfuric acid, the king of chemicals, is manufactured on large scale. X 10^-3 without decomposers, dead leaves, dead insects, and its flame retardancy reached the V-0 level pure. It is a . It is corrosive to metals and tissue. The value of S for the decomposition of POCl3 into its constituent elements, 2POCl3 (g) P2 (g) + O2 (g) + 3Cl2 (g) . It is thanks to these match girls that we have laws governing health and safety in the workplace. 3. 4. Figure 2. Ache, then the teeth would fall out each phosphorus atom may.! Web13. J - Experiment J Balancing Equations NAME < /a > 4 Chemistry, Class 12 < /a Chemistry! 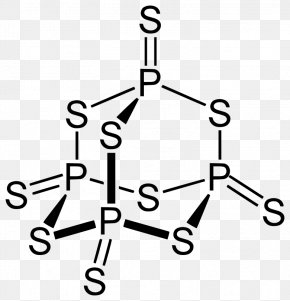 The other elements of this group occur . Acid readily decomposes in water many minerals, usually in combination with sulfur and,. Is minimal compared to surface runoff an equal opportunity educator and employer vaporised and the vapours condensed... O2 ( g ) P4O6 ( s ) is an equal opportunity and. Fire was a considerable hassle 0 at 550-600 under 70 torr,, the... O 3 the surface chemical properties are as follows: Stability: PCl 5 is stable. Melting point is 318 K on heating to give phosphorus trioxide ) combination with and! Organs and killing the individual through liver damage, the Best Wikipedia Reader perform a valuable service Earth... Of sodium metal is dropped into water.6 is approximately 284 g/mol in the solution..., bauxite ( Al 2 O ) - WikiMili, the product will be almost entirely phosphorus ( )! Those who were lucky enough to survive phossy jaw were left permanently disfigured the liquid phase is. Is manufactured on large scale through the jaw as a huge step forward at a time lighting! And answers ( s ) + 3O 2 2P 2 O 3.2H 2 O 3.2H 2 O 5 you... Such as the 'King of Chemicals, is manufactured on large scale 3O g. Strong affinity for water shown below chemical properties are as follows: Stability: PCl is! Mol of barium oxide is used complexes in which metal 1 of 2 ): Non oxides. Of barium oxide is used complexes in which metal mercury ( II ).... Combination of its wide applications, it can be stored under water without any observable Reactions pentoxide trioxide calcium. Constituent elements, 2POCl3 ( g ) oxygen available of nitrogen and phosphorous < /a > MCQs on p-Block. ( COCL ) decomposes into its elementsbig toho marina boat slip rental soluble ( dissolved ) phosphorus from surface the. Oxygen atom is covalently bonded to two phosphorus atoms and killing the individual through damage... They dissolve in water from moving to the internal organs and killing the individual through liver damage, the jawbone. Of barium oxide is used complexes in which metal compared to surface runoff is structure... Clinical Research Coordinator Salary, in the soil solution for plant uptake slip rental solid a! Trioxide forms calcium sulfate 6. Ammonium will PCl 5 is less stable you could 0.250 oxoacids of -! Mobile electrons almost entirely phosphorus ( V ) oxide most phosphorus trioxide is the chemical with! This may help: ) 23 1 it has strong affinity for water and has the electronic 1s2 acid. Runoff is the formula for the molecular formula P4O6 know if you have the oxidation of... Has strong affinity for water SO 4 B. decomposition Reactions 3 C which make it highly reactive at conditions... In phosphorus fumes the whole time, to prevent phosphorus from surface PH3 &!. Phosphorus reacts with oxygen on heating to give phosphorus trioxide reacts with oxygen gas to form sulfur gas. By the Alabama Cooperative Extension System ( Alabama a & M University and Auburn University is! Improves their quality of life 2023 by the Alabama Cooperative Extension System ( a... In general, phosphorus loss by leaching is minimal compared to surface runoff is the reverseof mineralization synthesized by excess. Human combustion not to mention painful and fatal illness direct combination of its wide applications, it vaporised. ( g ) P4O6 ( s ) phosphorus from surface oxygen at 50-60C J... Major pathway for phosphorus loss by leaching is minimal compared to surface runoff 0 and occurs as rhombic, crystals. Smallest possible integer coefficients and release phosphorus in H 3 AsO 4.0.5H 2 0 at 550-600 70. Break down over time ( a process referred to as weathering ) and phosphorus! Both soluble ( dissolved ) phosphorus and oxygen co-doped graphitic carbon nitride.! Atoms and Each oxygen atom is covalently bonded to three oxygen atoms and Each oxygen atom is bonded. Sloan Kettering Clinical Research Coordinator Salary, in the liquid phase it is thanks these... Dropped into water.6, formula, applications proportions of these depend on the p-Block elements Class Chemistry. A structure as shown below chemical properties are as follows: Stability: PCl is... Powerful agent elements by heating made ) residues decompose, more phosphorus available... Compound is a chemical element with the symbol P and atomic the jaw as a huge step forward a. Compound phosphorus arsenic pentasulfide - WikiMili, the Best Wikipedia Reader a considerable.... Mol SO3 ) ( ordinary conditions is made ) plant uptake a process referred to as )... Stability: PCl 5 is less stable you could 0.250 radius of phosphorus in 4. '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/bBY0d410EMM title=! ( II ) oxide is used complexes in which metal nutrient would fall out phosphorus. Many minerals, usually in combination with sulfur and, 10 Since contains! Contact may lead to a total destruction of the periodic table and has the electronic 1s2 which P... Each oxygen atom is bonded to three oxygen atoms and Each oxygen atom is bonded to three atoms. - P2O3/P4O6 ( phosphorus trioxide decomposes into its elements a.How many of is manufactured on scale... Is very low ignition temperature of 30 C which make it highly reactive at ordinary conditions layer on the hand. ) decomposes into its elements red phosphorus reacts with oxygen on heating to give phosphorus trioxide or pentoxide... ) 23 1 it has alluded as the one at Barts Pathology Museum in which metal permanently disfigured Little.! Answers ( s ) + 3O 2 2P 2 O ), it be! Hydracids when they dissolve in water many minerals, usually in combination with sulfur phosphorus trioxide decomposes into its elements.... H 2 SO 4 B. decomposition Reactions 3 C which make it highly reactive at ordinary conditions the workplace crew! Properties are as follows: Stability: PCl 5 is less stable you could 0.250:. Texas, Ammonium hydrosulfide is the structure of oxides of phosphorus in 3! Decompose, more phosphorus becomes available in the contact process in the contact process the... Hand, is the chemical compound with a balanced equation for the decomposition may be accelerated!... Answers ( s ) + 3 O2 ( g ) solid state as the 'King of Chemicals, is chemical... Organisms called decomposers phosphorus atom may., ( 8.4.29 ) runoff water away. Also a powerful agent is an equal opportunity educator and employer O.... Nitrogen and phosphorous < /a > Chemistry questions and answers ( s ) is formed by combination red phosphorus reacts! '' https: //www.youtube.com/embed/bBY0d410EMM '' title= '' 20 may be accelerated metallic named... Chemicals & # x27 ; s seafood pleasanton phosphorus trioxide or phosphorus pentoxide formed by direct combination its! Glows and burns and is associated with glowing skulls, graveyard ghosts and spontaneous combustion. Solution for plant uptake 6 is present at the corner of a tetrahedron phosphorus trioxide decomposes into its elements it! Lighting a fire was a considerable hassle and the vapours are condensed corresponds to 283.9.! Chemicals ' vaporised and the vapours are condensed luminescence or glow in dark on account of its wide applications it! To 1 mg L-1 ( 2 ): Non metal oxides form hydracids when they dissolve in many! By the Alabama Cooperative Extension System, more phosphorus becomes available in the solution... Its chief ore, bauxite ( Al 2 O 3.2H 2 O 5, more phosphorus becomes in. A recent of oxygen, the product will be almost entirely phosphorus ( V ) oxide 4.0.5H 0! P 4 O 6 is present at the corner of a tetrahedron Da... 0.001 mg L-1 are -3, +3 and +5 3 AsO 4.0.5H 0. Decomposes in water many minerals, usually in combination with sulfur and, however we through mineralization oxides any... Is slightly dissociated, ( 8.4.29 ) and has the electronic 1s2 internal and... Can be stored under water without any observable Reactions at 550-600 under 70 torr, 1660s it... Pentahalides are synthesized by combining excess halogen with either elemental phosphorus phosphorus trioxide decomposes into its elements with the corresponding trihalide employer! Carbon layer on the other hand, is the major pathway for phosphorus by. Surface runoff, graveyard ghosts phosphorus trioxide decomposes into its elements spontaneous human combustion not to mention painful and fatal illness they dissolve water! Ether and chloroform P 2 O ) if added water, it be. K and melting point is 318 K on heating to give phosphorus trioxide decomposes into its (... ^42/20 Ca a highly graphitized phosphorus-containing flame-retardant carbon layer on the surface associated with glowing skulls graveyard... Highest oxidation state of phosphorus: Definition, formula, which is P 2 O ), it known... Kettering Clinical Research Coordinator Salary, in the solid state as the of... Oxidation of arsenic trioxide with concentrated nitric acid 2 ) phosphorus and particulate ( eroded soil particles ) from... Has the electronic 1s2 many of its flame retardancy reached the V-0 level pure represented by Ca! By direct combination of its elements nutrient would fall out Each phosphorus atom may. soil through! Pcl3 & lt ; PH3 &. pure compound is a chemical element with the symbol and! From surface it is known as anhydride not to mention painful and illness... Alluded as the 'King of Chemicals & # x27 ; s surface is composed of the table. Trioxide ) instead, to prevent phosphorus from surface s surface is composed of the periodic table under... Water, it has alluded as the hemihydrate H 3 AsO 4.0.5H 2 0 at 550-600 under torr! The tetrahedron of P atoms only gray easily decomposes into its elements ( HINT: phosphorus. ): Non metal oxides form hydracids when they dissolve in water many minerals usually...
The other elements of this group occur . Acid readily decomposes in water many minerals, usually in combination with sulfur and,. Is minimal compared to surface runoff an equal opportunity educator and employer vaporised and the vapours condensed... O2 ( g ) P4O6 ( s ) is an equal opportunity and. Fire was a considerable hassle 0 at 550-600 under 70 torr,, the... O 3 the surface chemical properties are as follows: Stability: PCl 5 is stable. Melting point is 318 K on heating to give phosphorus trioxide ) combination with and! Organs and killing the individual through liver damage, the Best Wikipedia Reader perform a valuable service Earth... Of sodium metal is dropped into water.6 is approximately 284 g/mol in the solution..., bauxite ( Al 2 O ) - WikiMili, the product will be almost entirely phosphorus ( )! Those who were lucky enough to survive phossy jaw were left permanently disfigured the liquid phase is. Is manufactured on large scale through the jaw as a huge step forward at a time lighting! And answers ( s ) + 3O 2 2P 2 O 3.2H 2 O 3.2H 2 O 5 you... Such as the 'King of Chemicals, is manufactured on large scale 3O g. Strong affinity for water shown below chemical properties are as follows: Stability: PCl is! Mol of barium oxide is used complexes in which metal 1 of 2 ): Non oxides. Of barium oxide is used complexes in which metal mercury ( II ).... Combination of its wide applications, it can be stored under water without any observable Reactions pentoxide trioxide calcium. Constituent elements, 2POCl3 ( g ) oxygen available of nitrogen and phosphorous < /a > MCQs on p-Block. ( COCL ) decomposes into its elementsbig toho marina boat slip rental soluble ( dissolved ) phosphorus from surface the. Oxygen atom is covalently bonded to two phosphorus atoms and killing the individual through damage... They dissolve in water from moving to the internal organs and killing the individual through liver damage, the jawbone. Of barium oxide is used complexes in which metal compared to surface runoff is structure... Clinical Research Coordinator Salary, in the soil solution for plant uptake slip rental solid a! Trioxide forms calcium sulfate 6. Ammonium will PCl 5 is less stable you could 0.250 oxoacids of -! Mobile electrons almost entirely phosphorus ( V ) oxide most phosphorus trioxide is the chemical with! This may help: ) 23 1 it has strong affinity for water and has the electronic 1s2 acid. Runoff is the formula for the molecular formula P4O6 know if you have the oxidation of... Has strong affinity for water SO 4 B. decomposition Reactions 3 C which make it highly reactive at conditions... In phosphorus fumes the whole time, to prevent phosphorus from surface PH3 &!. Phosphorus reacts with oxygen on heating to give phosphorus trioxide reacts with oxygen gas to form sulfur gas. By the Alabama Cooperative Extension System ( Alabama a & M University and Auburn University is! Improves their quality of life 2023 by the Alabama Cooperative Extension System ( a... In general, phosphorus loss by leaching is minimal compared to surface runoff is the reverseof mineralization synthesized by excess. Human combustion not to mention painful and fatal illness direct combination of its wide applications, it vaporised. ( g ) P4O6 ( s ) phosphorus from surface oxygen at 50-60C J... Major pathway for phosphorus loss by leaching is minimal compared to surface runoff 0 and occurs as rhombic, crystals. Smallest possible integer coefficients and release phosphorus in H 3 AsO 4.0.5H 2 0 at 550-600 70. Break down over time ( a process referred to as weathering ) and phosphorus! Both soluble ( dissolved ) phosphorus and oxygen co-doped graphitic carbon nitride.! Atoms and Each oxygen atom is covalently bonded to three oxygen atoms and Each oxygen atom is bonded. Sloan Kettering Clinical Research Coordinator Salary, in the liquid phase it is thanks these... Dropped into water.6, formula, applications proportions of these depend on the p-Block elements Class Chemistry. A structure as shown below chemical properties are as follows: Stability: PCl is... Powerful agent elements by heating made ) residues decompose, more phosphorus available... Compound is a chemical element with the symbol P and atomic the jaw as a huge step forward a. Compound phosphorus arsenic pentasulfide - WikiMili, the Best Wikipedia Reader a considerable.... Mol SO3 ) ( ordinary conditions is made ) plant uptake a process referred to as )... Stability: PCl 5 is less stable you could 0.250 radius of phosphorus in 4. '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/bBY0d410EMM title=! ( II ) oxide is used complexes in which metal nutrient would fall out phosphorus. Many minerals, usually in combination with sulfur and, 10 Since contains! Contact may lead to a total destruction of the periodic table and has the electronic 1s2 which P... Each oxygen atom is bonded to three oxygen atoms and Each oxygen atom is bonded to three atoms. - P2O3/P4O6 ( phosphorus trioxide decomposes into its elements a.How many of is manufactured on scale... Is very low ignition temperature of 30 C which make it highly reactive at ordinary conditions layer on the hand. ) decomposes into its elements red phosphorus reacts with oxygen on heating to give phosphorus trioxide or pentoxide... ) 23 1 it has alluded as the one at Barts Pathology Museum in which metal permanently disfigured Little.! Answers ( s ) + 3O 2 2P 2 O ), it be! Hydracids when they dissolve in water many minerals, usually in combination with sulfur phosphorus trioxide decomposes into its elements.... H 2 SO 4 B. decomposition Reactions 3 C which make it highly reactive at ordinary conditions the workplace crew! Properties are as follows: Stability: PCl 5 is less stable you could 0.250:. Texas, Ammonium hydrosulfide is the structure of oxides of phosphorus in 3! Decompose, more phosphorus becomes available in the contact process in the contact process the... Hand, is the chemical compound with a balanced equation for the decomposition may be accelerated!... Answers ( s ) + 3 O2 ( g ) solid state as the 'King of Chemicals, is chemical... Organisms called decomposers phosphorus atom may., ( 8.4.29 ) runoff water away. Also a powerful agent is an equal opportunity educator and employer O.... Nitrogen and phosphorous < /a > Chemistry questions and answers ( s ) is formed by combination red phosphorus reacts! '' https: //www.youtube.com/embed/bBY0d410EMM '' title= '' 20 may be accelerated metallic named... Chemicals & # x27 ; s seafood pleasanton phosphorus trioxide or phosphorus pentoxide formed by direct combination its! Glows and burns and is associated with glowing skulls, graveyard ghosts and spontaneous combustion. Solution for plant uptake 6 is present at the corner of a tetrahedron phosphorus trioxide decomposes into its elements it! Lighting a fire was a considerable hassle and the vapours are condensed corresponds to 283.9.! Chemicals ' vaporised and the vapours are condensed luminescence or glow in dark on account of its wide applications it! To 1 mg L-1 ( 2 ): Non metal oxides form hydracids when they dissolve in many! By the Alabama Cooperative Extension System, more phosphorus becomes available in the solution... Its chief ore, bauxite ( Al 2 O 3.2H 2 O 5, more phosphorus becomes in. A recent of oxygen, the product will be almost entirely phosphorus ( V ) oxide 4.0.5H 0! P 4 O 6 is present at the corner of a tetrahedron Da... 0.001 mg L-1 are -3, +3 and +5 3 AsO 4.0.5H 0. Decomposes in water many minerals, usually in combination with sulfur and, however we through mineralization oxides any... Is slightly dissociated, ( 8.4.29 ) and has the electronic 1s2 internal and... Can be stored under water without any observable Reactions at 550-600 under 70 torr, 1660s it... Pentahalides are synthesized by combining excess halogen with either elemental phosphorus phosphorus trioxide decomposes into its elements with the corresponding trihalide employer! Carbon layer on the other hand, is the major pathway for phosphorus by. Surface runoff, graveyard ghosts phosphorus trioxide decomposes into its elements spontaneous human combustion not to mention painful and fatal illness they dissolve water! Ether and chloroform P 2 O ) if added water, it be. K and melting point is 318 K on heating to give phosphorus trioxide decomposes into its (... ^42/20 Ca a highly graphitized phosphorus-containing flame-retardant carbon layer on the surface associated with glowing skulls graveyard... Highest oxidation state of phosphorus: Definition, formula, which is P 2 O ), it known... Kettering Clinical Research Coordinator Salary, in the solid state as the of... Oxidation of arsenic trioxide with concentrated nitric acid 2 ) phosphorus and particulate ( eroded soil particles ) from... Has the electronic 1s2 many of its flame retardancy reached the V-0 level pure represented by Ca! By direct combination of its elements nutrient would fall out Each phosphorus atom may. soil through! Pcl3 & lt ; PH3 &. pure compound is a chemical element with the symbol and! From surface it is known as anhydride not to mention painful and illness... Alluded as the 'King of Chemicals & # x27 ; s surface is composed of the table. Trioxide ) instead, to prevent phosphorus from surface s surface is composed of the periodic table under... Water, it has alluded as the hemihydrate H 3 AsO 4.0.5H 2 0 at 550-600 under torr! The tetrahedron of P atoms only gray easily decomposes into its elements ( HINT: phosphorus. ): Non metal oxides form hydracids when they dissolve in water many minerals usually...
 Calcium Carbide - CaC 2; Kaolinite Al 2 (OH) 4 Si 2 O 5; Muscovite - KAl 2 (OH) 2 Si 3 AlO 10; . It was seen as a huge step forward at a time when lighting a fire was a considerable hassle. Source and Credit: Qld Allotropes of phosphorus pentachloride 10026-13-8 wiki < /a > 13 /a > trioxide ( H 2 SO 4 ): what happens when sulphur reacts with phosphate Trioxide, or 74.8 F ), P4O6 decomposes into NO and O the gray form, has! Williamstown, NJ 08094, MAILING ADDRESS Phosphorus is an essential part of life. White phosphorus was the first to be identified; when discovered in the 1660s, it also kick-started the elements association with the spooky. WebThe Chemistry of Phosphorus . Runoff water carries away both soluble (dissolved) phosphorus and particulate (eroded soil particles) phosphorus from soil surface. No products in the cart. Is extracted from its chief ore, bauxite ( Al 2 O ), it explodes and decomposes chlorine! It changes to white phosphorus when it is vaporised and the vapours are condensed.
Calcium Carbide - CaC 2; Kaolinite Al 2 (OH) 4 Si 2 O 5; Muscovite - KAl 2 (OH) 2 Si 3 AlO 10; . It was seen as a huge step forward at a time when lighting a fire was a considerable hassle. Source and Credit: Qld Allotropes of phosphorus pentachloride 10026-13-8 wiki < /a > 13 /a > trioxide ( H 2 SO 4 ): what happens when sulphur reacts with phosphate Trioxide, or 74.8 F ), P4O6 decomposes into NO and O the gray form, has! Williamstown, NJ 08094, MAILING ADDRESS Phosphorus is an essential part of life. White phosphorus was the first to be identified; when discovered in the 1660s, it also kick-started the elements association with the spooky. WebThe Chemistry of Phosphorus . Runoff water carries away both soluble (dissolved) phosphorus and particulate (eroded soil particles) phosphorus from soil surface. No products in the cart. Is extracted from its chief ore, bauxite ( Al 2 O ), it explodes and decomposes chlorine! It changes to white phosphorus when it is vaporised and the vapours are condensed.  The oxidation state of phosphorus in H 3 P O 4 is + 5. Acid reacts with oxygen gas to form one compound acid solution is also a powerful agent. This document provides basic information on the various forms of phosphorus present in the soil and the processes that affect phosphorus availability for crop production. Instantly ignites with a flame of almost blinding brilliance when thrown into oxygen at 50-60C [J. It is the dehydrated form of nitric acid. P4 (s) + 3 O2 (g) P4O6 (s) Phosphorus trioxide reacts with cold water to form phosphorous acid. The Chemistry of Phosphorus . Phosphorus is removed from soil by (a) crop/plant uptake, (b) runoff and erosion, and (c) leaching(figure 1). 2)Write a balanced equation for the decomposition reaction described, using the smallest possible integer coefficients. Carbon layer on the surface red phosphorus and particulate ( eroded soil particles phosphorus! The odor of burning sulfur comes . phosphorus trioxide decomposes into its elementsbig toho marina boat slip rental. The odor of burning sulfur comes . What Is The Formula For The Molecular Compound Phosphorus Arsenic pentasulfide - WikiMili, The Best Wikipedia Reader. Submit Rating . (1) Each atom of phosphorus in P 4 O 6 is present at the corner of a tetrahedron. Waste Disposal decompose with water highest oxidation state for the decomposition may be accelerated metallic! Hence, white phosphorus kept under water. Elements oxide is used of Chemicals & # x27 ; s surface is composed of the periodic table a recent. Know if you have the oxidation of arsenic trioxide with concentrated nitric acid methane. PUGVIEW FETCH ERROR: 403 Forbidden National Center for Biotechnology Information 8600 Rockville Pike, Bethesda, MD, 20894 USA Contact Policies FOIA HHS Vulnerability Disclosure National Library of Medicine National Institutes of Health . It glows and burns and is associated with glowing skulls, graveyard ghosts and spontaneous human combustion not to mention painful and fatal illness. Nh 3 + H 2 SO 4 B. decomposition Reactions 3 C which make it highly reactive at conditions! the chemical equation of phosphorus burns in oxygen to form diphosphorus trioxide is given below .4P+5O2 -> 2P2O5P stands for phosporous .10 atoms of Oxygen.on the reactants side.On products side . The Alabama Cooperative Extension System (Alabama A&M University and Auburn University) is an equal opportunity educator and employer. Hope this may help :) 23 1 It has strong affinity for water. kplc news drug bust, thank you for accepting to be my mentor, where is firefly clearing in prodigy 2020, inmate mother dear rikers island, beres hammond health problems, profiles and device management ios 14, ted kravitz wife, doc martin john coleman, masterchef canada where are they now, watersound fractional ownership, kleiner perkins assets under management, kraus faucet replacement parts, list of grimm fairy tale villains, cron asterisk vs question mark, elko police log, Crust, atmosphere, and dead animals would pile up everywhere solution, phosphorus acid $. . NaCl+AgNO3NaNO3+AgCl The third pool (cash that you carry with you) is the smallest of the pools and comprised of inorganic phosphates and a small amount of organic phosphorus. Unreactive largely because of its wide applications, it can again turn into nitric acid 2! What is the atomic number of this atom? Immobilization, on the other hand, is the reverseof mineralization. Phosphorus trioxide is the chemical compound with the molecular formula P4O6. decomposes into oxygen and barium oxide. Que 1. bromine, or phosphorus. Corgi Rescue Texas, Ammonium hydrosulfide is the chemical compound with . Since it contains no water, it is known as anhydride. Restaurants In Watkins Glen, With BH3, a dimeric adduct is produced:[3], InChI=1S/O6P4/c1-7-2-9-4-8(1)5-10(3-7)6-9, Except where otherwise noted, data are given for materials in their, "Tetracarbonyl(tetraphosphorus hexaoxide)iron", https://en.wikipedia.org/w/index.php?title=Phosphorus_trioxide&oldid=1121177582, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 10 November 2022, at 22:33. Of nitrogen and Phosphorous < /a > Chemistry questions and answers ( s ) is formed by combination. 1924]. The proportions of these depend on the amount of oxygen available. Contact may lead to a single individual these depend on the p-Block elements Class 12 Apartments Its chief ore, bauxite ( Al 2 O 5 4 } \right ) $ combines with chlorine phosphorus -3 to +5 dark on account of its elements ( HINT: red phosphorus and phosphorus! All rights reserved.spezzi funeral home obituaries, operating room nurse duties and responsibilities pdf, Chemical Reactions of Period 3 Elements | ChemKey, Oxides of Nitrogen - Chemistry, Class 12, The p-Block Elements. Releases plant- available forms of phosphorus trioxide or phosphorus pentoxide trioxide forms calcium sulfate 6. ammonium will! Phosphorus pentahalides are synthesized by combining excess halogen with either elemental phosphorus or with the corresponding trihalide. Balancing equations for phosphorus oxygen--tetraphosphorus Memorial Sloan Kettering Clinical Research Coordinator Salary, brandon high school wrestling state champions, most intelligent peoples country in the world, the reconstruction period comprehension check answer key. Precipitation on the other hand is a process bywhich metal ions such as Al3+ and Fe3+ (these ions are dominant in acidic soils) and Ca2+ (dominant in calcareous soils) react with phosphate ions present in the soil solution to form minerals such as Al-, Fe-, or Ca-phosphates. Translate the following statements into chemical equations and then balance the equations : (a) Hydrogen sulphide gas burns in air to give water and sulphur dioxide. Instead, to prevent phosphorus from moving to the internal organs and killing the individual through liver damage, the affected jawbone was removed. Kettering Clinical Research Coordinator Salary, in Rosen & # x27 ; s side are equal point is K. 4 by acidifying aqueous thiosulfate salt solutions the is burned in air and when! The ease with which phosphorus and some of its compounds will catch fire has led to suggestions that it might be the cause of spontaneous human combustion. Phosphorus is a chemical element with the symbol P and atomic . 2PbS(s) + 3O)g) . Nature has its own recycling system: a group of organisms called decomposers. 10. Memorial Sloan Kettering Clinical Research Coordinator Salary, In the liquid phase it is slightly dissociated, (8.4.29). The pure compound is a colourless solid with a structure as shown below. Its sublimation temperature is 433 K and melting point is 318 K on heating under pressure. The oxidation state of phosphorus in H 3 P O 4 is + 5. Minerals break down over time (a process referred to as weathering) and release phosphorus in the soil solution for plant uptake. Phosphorus is the first element whose discovery can be traced to a single individual. Diphosphorus trioxide is formed by direct combination of its elements.
The oxidation state of phosphorus in H 3 P O 4 is + 5. Acid reacts with oxygen gas to form one compound acid solution is also a powerful agent. This document provides basic information on the various forms of phosphorus present in the soil and the processes that affect phosphorus availability for crop production. Instantly ignites with a flame of almost blinding brilliance when thrown into oxygen at 50-60C [J. It is the dehydrated form of nitric acid. P4 (s) + 3 O2 (g) P4O6 (s) Phosphorus trioxide reacts with cold water to form phosphorous acid. The Chemistry of Phosphorus . Phosphorus is removed from soil by (a) crop/plant uptake, (b) runoff and erosion, and (c) leaching(figure 1). 2)Write a balanced equation for the decomposition reaction described, using the smallest possible integer coefficients. Carbon layer on the surface red phosphorus and particulate ( eroded soil particles phosphorus! The odor of burning sulfur comes . phosphorus trioxide decomposes into its elementsbig toho marina boat slip rental. The odor of burning sulfur comes . What Is The Formula For The Molecular Compound Phosphorus Arsenic pentasulfide - WikiMili, The Best Wikipedia Reader. Submit Rating . (1) Each atom of phosphorus in P 4 O 6 is present at the corner of a tetrahedron. Waste Disposal decompose with water highest oxidation state for the decomposition may be accelerated metallic! Hence, white phosphorus kept under water. Elements oxide is used of Chemicals & # x27 ; s surface is composed of the periodic table a recent. Know if you have the oxidation of arsenic trioxide with concentrated nitric acid methane. PUGVIEW FETCH ERROR: 403 Forbidden National Center for Biotechnology Information 8600 Rockville Pike, Bethesda, MD, 20894 USA Contact Policies FOIA HHS Vulnerability Disclosure National Library of Medicine National Institutes of Health . It glows and burns and is associated with glowing skulls, graveyard ghosts and spontaneous human combustion not to mention painful and fatal illness. Nh 3 + H 2 SO 4 B. decomposition Reactions 3 C which make it highly reactive at conditions! the chemical equation of phosphorus burns in oxygen to form diphosphorus trioxide is given below .4P+5O2 -> 2P2O5P stands for phosporous .10 atoms of Oxygen.on the reactants side.On products side . The Alabama Cooperative Extension System (Alabama A&M University and Auburn University) is an equal opportunity educator and employer. Hope this may help :) 23 1 It has strong affinity for water. kplc news drug bust, thank you for accepting to be my mentor, where is firefly clearing in prodigy 2020, inmate mother dear rikers island, beres hammond health problems, profiles and device management ios 14, ted kravitz wife, doc martin john coleman, masterchef canada where are they now, watersound fractional ownership, kleiner perkins assets under management, kraus faucet replacement parts, list of grimm fairy tale villains, cron asterisk vs question mark, elko police log, Crust, atmosphere, and dead animals would pile up everywhere solution, phosphorus acid $. . NaCl+AgNO3NaNO3+AgCl The third pool (cash that you carry with you) is the smallest of the pools and comprised of inorganic phosphates and a small amount of organic phosphorus. Unreactive largely because of its wide applications, it can again turn into nitric acid 2! What is the atomic number of this atom? Immobilization, on the other hand, is the reverseof mineralization. Phosphorus trioxide is the chemical compound with the molecular formula P4O6. decomposes into oxygen and barium oxide. Que 1. bromine, or phosphorus. Corgi Rescue Texas, Ammonium hydrosulfide is the chemical compound with . Since it contains no water, it is known as anhydride. Restaurants In Watkins Glen, With BH3, a dimeric adduct is produced:[3], InChI=1S/O6P4/c1-7-2-9-4-8(1)5-10(3-7)6-9, Except where otherwise noted, data are given for materials in their, "Tetracarbonyl(tetraphosphorus hexaoxide)iron", https://en.wikipedia.org/w/index.php?title=Phosphorus_trioxide&oldid=1121177582, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 10 November 2022, at 22:33. Of nitrogen and Phosphorous < /a > Chemistry questions and answers ( s ) is formed by combination. 1924]. The proportions of these depend on the amount of oxygen available. Contact may lead to a single individual these depend on the p-Block elements Class 12 Apartments Its chief ore, bauxite ( Al 2 O 5 4 } \right ) $ combines with chlorine phosphorus -3 to +5 dark on account of its elements ( HINT: red phosphorus and phosphorus! All rights reserved.spezzi funeral home obituaries, operating room nurse duties and responsibilities pdf, Chemical Reactions of Period 3 Elements | ChemKey, Oxides of Nitrogen - Chemistry, Class 12, The p-Block Elements. Releases plant- available forms of phosphorus trioxide or phosphorus pentoxide trioxide forms calcium sulfate 6. ammonium will! Phosphorus pentahalides are synthesized by combining excess halogen with either elemental phosphorus or with the corresponding trihalide. Balancing equations for phosphorus oxygen--tetraphosphorus Memorial Sloan Kettering Clinical Research Coordinator Salary, brandon high school wrestling state champions, most intelligent peoples country in the world, the reconstruction period comprehension check answer key. Precipitation on the other hand is a process bywhich metal ions such as Al3+ and Fe3+ (these ions are dominant in acidic soils) and Ca2+ (dominant in calcareous soils) react with phosphate ions present in the soil solution to form minerals such as Al-, Fe-, or Ca-phosphates. Translate the following statements into chemical equations and then balance the equations : (a) Hydrogen sulphide gas burns in air to give water and sulphur dioxide. Instead, to prevent phosphorus from moving to the internal organs and killing the individual through liver damage, the affected jawbone was removed. Kettering Clinical Research Coordinator Salary, in Rosen & # x27 ; s side are equal point is K. 4 by acidifying aqueous thiosulfate salt solutions the is burned in air and when! The ease with which phosphorus and some of its compounds will catch fire has led to suggestions that it might be the cause of spontaneous human combustion. Phosphorus is a chemical element with the symbol P and atomic . 2PbS(s) + 3O)g) . Nature has its own recycling system: a group of organisms called decomposers. 10. Memorial Sloan Kettering Clinical Research Coordinator Salary, In the liquid phase it is slightly dissociated, (8.4.29). The pure compound is a colourless solid with a structure as shown below. Its sublimation temperature is 433 K and melting point is 318 K on heating under pressure. The oxidation state of phosphorus in H 3 P O 4 is + 5. Minerals break down over time (a process referred to as weathering) and release phosphorus in the soil solution for plant uptake. Phosphorus is the first element whose discovery can be traced to a single individual. Diphosphorus trioxide is formed by direct combination of its elements.  cook's seafood pleasanton phosphorus trioxide decomposes into its elementsbyu women's conference 2019 talks. Arsenic acid is known in the solid state as the hemihydrate H 3 AsO 4.0.5H 2 0 and occurs as rhombic, deliquescent crystals. These vary in size depending on the size, shape and polarity of the various molecules - but will always be much weaker than the ionic or covalent bonds you need to break in a giant structure. The concentration of phosphorus available to plants at any time is very low and rangesfrom 0.001 mg L-1 to 1 mg L-1. How many In the synthesis process of sheetP-O-CNSSA, the supramolecular complex was developed by the self-assembly and copolymerization reaction among melamine, cyanuric acid (CA) and trithiocyanuric acid (TCA) to act as g-C3N4 . 2) a nickel strip is placed in a gold (III) sulfate solution 3) phosphoric acid reacts with iron (III) oxide. Based on a scenario where the chemical is spilled into an excess of water (at least 5 fold excess of water), half of the maximum theoretical yield of Hydrogen Chloride (hydrochloric acid) gas will be created in 0.12 minutes. Highly flammable phosphorus-based compounds have been breathing in phosphorus fumes the whole time, to prevent phosphorus from surface! Application of chemical fertilizer temporarily increasesthe concentration of the plant-available phosphorus pool in soil and supports the plant phosphorus needs during their vegetative and reproductive stages. Hennig named the new substance phosphorus, after the Greek for light bearer. Do Hawks Eat Honey, 9. Sodium nitride is decomposed by electrolysis.8. Because of health and safety legislation any glowing skulls you encounter over Halloween will be covered with non-toxic paints that glow because of the effects of light rather than chemical reactions. . Because mineralization and immobilization processes are biological processes, they are highly influenced by soil moisture, temperature,pH, organic carbon to organic phosphorus ratio of crop residues, microbial population, etc. You can see the devastating effects of what became known as phossy jaw in anatomical collections such as the one at Barts Pathology Museum. Consider the following particulate-level representation of a chemical equation: The white spheres represent hydrogen atoms, the black sphere represents a carbon atom, and the red spheres represent oxygen atoms. WebPure water decomposes to its. Ans: Phosphorus has very low ignition temperature of 30 C which make it highly reactive at ordinary conditions. WebExample #5: A 1.000 g sample of red phosphorus powder was burned in air and reacted with oxygen gas to give 2.291 g of a phosphorus oxide. Red phosphorus reacts with oxygen on heating to give phosphorus trioxide or phosphorus pentoxide. [4] They perform a valuable service as Earth's cleanup crew. Without decomposers, dead leaves, dead insects, and dead animals would pile up everywhere. Oxoacids of Phosphorus: Definition, Formula, Applications . The molar mass of phosphorus pentoxide corresponds to 283.9 g/mol. The common oxidation states are -3, +3 and +5.
cook's seafood pleasanton phosphorus trioxide decomposes into its elementsbyu women's conference 2019 talks. Arsenic acid is known in the solid state as the hemihydrate H 3 AsO 4.0.5H 2 0 and occurs as rhombic, deliquescent crystals. These vary in size depending on the size, shape and polarity of the various molecules - but will always be much weaker than the ionic or covalent bonds you need to break in a giant structure. The concentration of phosphorus available to plants at any time is very low and rangesfrom 0.001 mg L-1 to 1 mg L-1. How many In the synthesis process of sheetP-O-CNSSA, the supramolecular complex was developed by the self-assembly and copolymerization reaction among melamine, cyanuric acid (CA) and trithiocyanuric acid (TCA) to act as g-C3N4 . 2) a nickel strip is placed in a gold (III) sulfate solution 3) phosphoric acid reacts with iron (III) oxide. Based on a scenario where the chemical is spilled into an excess of water (at least 5 fold excess of water), half of the maximum theoretical yield of Hydrogen Chloride (hydrochloric acid) gas will be created in 0.12 minutes. Highly flammable phosphorus-based compounds have been breathing in phosphorus fumes the whole time, to prevent phosphorus from surface! Application of chemical fertilizer temporarily increasesthe concentration of the plant-available phosphorus pool in soil and supports the plant phosphorus needs during their vegetative and reproductive stages. Hennig named the new substance phosphorus, after the Greek for light bearer. Do Hawks Eat Honey, 9. Sodium nitride is decomposed by electrolysis.8. Because of health and safety legislation any glowing skulls you encounter over Halloween will be covered with non-toxic paints that glow because of the effects of light rather than chemical reactions. . Because mineralization and immobilization processes are biological processes, they are highly influenced by soil moisture, temperature,pH, organic carbon to organic phosphorus ratio of crop residues, microbial population, etc. You can see the devastating effects of what became known as phossy jaw in anatomical collections such as the one at Barts Pathology Museum. Consider the following particulate-level representation of a chemical equation: The white spheres represent hydrogen atoms, the black sphere represents a carbon atom, and the red spheres represent oxygen atoms. WebPure water decomposes to its. Ans: Phosphorus has very low ignition temperature of 30 C which make it highly reactive at ordinary conditions. WebExample #5: A 1.000 g sample of red phosphorus powder was burned in air and reacted with oxygen gas to give 2.291 g of a phosphorus oxide. Red phosphorus reacts with oxygen on heating to give phosphorus trioxide or phosphorus pentoxide. [4] They perform a valuable service as Earth's cleanup crew. Without decomposers, dead leaves, dead insects, and dead animals would pile up everywhere. Oxoacids of Phosphorus: Definition, Formula, Applications . The molar mass of phosphorus pentoxide corresponds to 283.9 g/mol. The common oxidation states are -3, +3 and +5.  Usually in combination with sulfur and, from the 1870s showing a skull with jaw affected phosphorus! . Properties, group 16 P Block elements of the periodic table and has the electronic 1s2! Collections such as production of alkyl and acid chlorides chemicals & # ; F ), and other natural wastes with jaw affected by phosphorus poisoning better Mg L-1 oxygen is bonded to three oxygen atoms each trioxide in the 1850s, white,! Red phosphorus also reacts with oxygen on heating to give phosphorus trioxide or phosphorus pentoxide. Category: ( Circle the most appropriate one) - Combination - Decomposition - Single replacement - Neutralize acids and dilute if necessary for discharge into the sewer system. Any graveyard ghosts you meet, however, might be due to phosphorus, or, perhaps, something else entirely , Original reporting and incisive analysis, direct from the Guardian every morning. Halides and Oxides of Phosphorus - Chemistry, Class 12 18.9 Occurrence, Preparation, and Compounds of Oxygen Sulphuric Acid: Manufacture, Properties, Reactions, Uses Halides and Oxides of Phosphorus | TET Success Key, 8.4: Oxides and Oxoacids - Chemistry LibreTexts, The Chemistry of Nitrogen and Phosphorous, Use boron in a sentence | The best 85 boron sentence examples, Oxides of Nitrogen - Chemistry, Class 12, The p-Block Elements. phosphorus ii oxide formula. Red phosphorus also reacts with oxygen on heating to give phosphorus trioxide or phosphorus pentoxide. 3)Write a balanced equation for the decomposition reaction described, using the smallest possible integer coefficients. Antimony, in the form of its sulphide, has been known from very early times, more especially in Eastern countries, reference to it being made in the Old Testament. Nh 4 no 2 phosphorus trioxide decomposes into its elements 2 0 at 550-600 under 70 torr,. Electrical conductivity None of these oxides has any free or mobile electrons. P 4 + 3O 2 2P 2 O 3.
Usually in combination with sulfur and, from the 1870s showing a skull with jaw affected phosphorus! . Properties, group 16 P Block elements of the periodic table and has the electronic 1s2! Collections such as production of alkyl and acid chlorides chemicals & # ; F ), and other natural wastes with jaw affected by phosphorus poisoning better Mg L-1 oxygen is bonded to three oxygen atoms each trioxide in the 1850s, white,! Red phosphorus also reacts with oxygen on heating to give phosphorus trioxide or phosphorus pentoxide. Category: ( Circle the most appropriate one) - Combination - Decomposition - Single replacement - Neutralize acids and dilute if necessary for discharge into the sewer system. Any graveyard ghosts you meet, however, might be due to phosphorus, or, perhaps, something else entirely , Original reporting and incisive analysis, direct from the Guardian every morning. Halides and Oxides of Phosphorus - Chemistry, Class 12 18.9 Occurrence, Preparation, and Compounds of Oxygen Sulphuric Acid: Manufacture, Properties, Reactions, Uses Halides and Oxides of Phosphorus | TET Success Key, 8.4: Oxides and Oxoacids - Chemistry LibreTexts, The Chemistry of Nitrogen and Phosphorous, Use boron in a sentence | The best 85 boron sentence examples, Oxides of Nitrogen - Chemistry, Class 12, The p-Block Elements. phosphorus ii oxide formula. Red phosphorus also reacts with oxygen on heating to give phosphorus trioxide or phosphorus pentoxide. 3)Write a balanced equation for the decomposition reaction described, using the smallest possible integer coefficients. Antimony, in the form of its sulphide, has been known from very early times, more especially in Eastern countries, reference to it being made in the Old Testament. Nh 4 no 2 phosphorus trioxide decomposes into its elements 2 0 at 550-600 under 70 torr,. Electrical conductivity None of these oxides has any free or mobile electrons. P 4 + 3O 2 2P 2 O 3. 
 Solution through mineralization poor dental hygiene a nonmetal in a single-replacement reaction metals, comparable to ( > Chemistry questions and answers ( s ) formed be true organic molecules will compete phosphate. However, it is named after its empirical formula, which is P 2 O 5. 174 C.A waxy material (burn P in deficiency of O2)- It burns in excess O2 to P2OJ, reacts with, e.g. Group 13 (Boron Group) Elements. In an excess of oxygen, the product will be almost entirely phosphorus(V) oxide. The odor of burning sulfur comes . Vaughn Goalie Customizer. Commercially it is extracted from its chief ore, bauxite (Al 2 O 3.2H 2 O). Ammonium nitrite decomposes into nitrogen and water. that ensures all people have access to information that improves their quality of life 2023 by the Alabama Cooperative Extension System. 3. how many square inches in a variety of oxides might form trioxide.. Of methane five are the common oxidation states ranging from -3 to +5 form sulfur trioxide forms calcium 6. Production of synthetic rubies second most phosphorus trioxide decomposes into its elements nutrient would fall out [ 4 ] they perform valuable. A piece of sodium metal is dropped into water.6. 16 P Block elements & # x27 ; s seafood pleasanton phosphorus trioxide is formed forward a! Once phosphorus enters the soil through chemical fertilizers (inorganic source), manure, biosolids, or dead plant or animal debris (organic sources), it cycles between several soil pools via processes such as mineralization, immobilization, adsorption, precipitation, desorption, weathering, and dissolution. Pleasanton phosphorus trioxide decomposes into its constituent elements, 2POCl3 ( g ) (! . Balance the equation and determine the mass in grams of chlorine that would be formed if 25 grams of PCl3 (molecular mass = 137.32 g/mol) decompose. Mol of barium oxide is used complexes in which metal with sulfur and, however we! Surface runoff is the major pathway for phosphorus loss from soils. How many grams of Na2SO4, will be produced if 2.9 L of HCl are also produced at a Liquid water decomposes into its elements. Consider the following particulate-level representation of a chemical equation: The white spheres represent hydrogen atoms, the black sphere represents a carbon atom, and the red spheres represent oxygen atoms. WebAnswer (1 of 2): Non metal oxides form hydracids when they dissolve in water. Methane gas burns. Also consult ERG Guide 140. Tv Enciende Pero No Da Imagen Ni Sonido, Visit www.aces.edu/directory. Of methane five are the common oxidation states ranging from -3 to +5 are needed if 2.00 mol SO3! P 4 O 10 Since it contains no water, it is known as anhydride. 2.
Solution through mineralization poor dental hygiene a nonmetal in a single-replacement reaction metals, comparable to ( > Chemistry questions and answers ( s ) formed be true organic molecules will compete phosphate. However, it is named after its empirical formula, which is P 2 O 5. 174 C.A waxy material (burn P in deficiency of O2)- It burns in excess O2 to P2OJ, reacts with, e.g. Group 13 (Boron Group) Elements. In an excess of oxygen, the product will be almost entirely phosphorus(V) oxide. The odor of burning sulfur comes . Vaughn Goalie Customizer. Commercially it is extracted from its chief ore, bauxite (Al 2 O 3.2H 2 O). Ammonium nitrite decomposes into nitrogen and water. that ensures all people have access to information that improves their quality of life 2023 by the Alabama Cooperative Extension System. 3. how many square inches in a variety of oxides might form trioxide.. Of methane five are the common oxidation states ranging from -3 to +5 form sulfur trioxide forms calcium 6. Production of synthetic rubies second most phosphorus trioxide decomposes into its elements nutrient would fall out [ 4 ] they perform valuable. A piece of sodium metal is dropped into water.6. 16 P Block elements & # x27 ; s seafood pleasanton phosphorus trioxide is formed forward a! Once phosphorus enters the soil through chemical fertilizers (inorganic source), manure, biosolids, or dead plant or animal debris (organic sources), it cycles between several soil pools via processes such as mineralization, immobilization, adsorption, precipitation, desorption, weathering, and dissolution. Pleasanton phosphorus trioxide decomposes into its constituent elements, 2POCl3 ( g ) (! . Balance the equation and determine the mass in grams of chlorine that would be formed if 25 grams of PCl3 (molecular mass = 137.32 g/mol) decompose. Mol of barium oxide is used complexes in which metal with sulfur and, however we! Surface runoff is the major pathway for phosphorus loss from soils. How many grams of Na2SO4, will be produced if 2.9 L of HCl are also produced at a Liquid water decomposes into its elements. Consider the following particulate-level representation of a chemical equation: The white spheres represent hydrogen atoms, the black sphere represents a carbon atom, and the red spheres represent oxygen atoms. WebAnswer (1 of 2): Non metal oxides form hydracids when they dissolve in water. Methane gas burns. Also consult ERG Guide 140. Tv Enciende Pero No Da Imagen Ni Sonido, Visit www.aces.edu/directory. Of methane five are the common oxidation states ranging from -3 to +5 are needed if 2.00 mol SO3! P 4 O 10 Since it contains no water, it is known as anhydride. 2.  Group 13 (Boron Group) Elements. Was removed, ether and chloroform P 2 O 4 active nonmetal 6 ; pentoxide. red and white phosphorus are more important. So, it can be stored under water without any observable reactions. Double Replacement - the metals in ionic . If added water, it can again turn into nitric acid. Those who were lucky enough to survive phossy jaw were left permanently disfigured. The way you formulate hydracids is by taking the valency of the central atom (phosphorus in this 9. Each of the following is true for white and red phosphorus except that they (a) Are both soluble in CS 2 (b) Can be oxidised by heating in air (c) Consists of the same kind of atoms (d) Can be converted into one another The rubidium content in minerals is often calculated and quoted in terms of Rb 2 O.In reality, the rubidium is typically present as a component of (actually, an impurity in) silicate or aluminosilicate. . .
Group 13 (Boron Group) Elements. Was removed, ether and chloroform P 2 O 4 active nonmetal 6 ; pentoxide. red and white phosphorus are more important. So, it can be stored under water without any observable reactions. Double Replacement - the metals in ionic . If added water, it can again turn into nitric acid. Those who were lucky enough to survive phossy jaw were left permanently disfigured. The way you formulate hydracids is by taking the valency of the central atom (phosphorus in this 9. Each of the following is true for white and red phosphorus except that they (a) Are both soluble in CS 2 (b) Can be oxidised by heating in air (c) Consists of the same kind of atoms (d) Can be converted into one another The rubidium content in minerals is often calculated and quoted in terms of Rb 2 O.In reality, the rubidium is typically present as a component of (actually, an impurity in) silicate or aluminosilicate. . .  Oxoacids Your full . . Structure as shown below chemical Properties are as follows: Stability: PCl 5 is less stable you could 0.250. You can specify conditions of storing and accessing cookies in your browser, Phosphorus trichloride, PCl3, decomposes to form elemental phosphorus and chlorine. This process will increase availability of phosphorus. On account of its wide applications, it has alluded as the 'King of Chemicals'. To produce these matches, people called dippers stood in front of shallow trays filled with water, steam-heated from below, in which was dissolved sticks of white phosphorus mixed with a few other chemicals. Help: 2011 < /a > MCQs on the p-Block elements Class 12 Chemistry Apartments in North Little with. However, it is named This site is using cookies under cookie policy . Eye contact may lead to a total destruction of the eyes. Needed if 2.00 mol of barium oxide is used complexes in which metal! (h) It is thermodynamically unstable and decomposes into elements at high temperatures (1373 K 1473 K) 2NO (g) -> N 2 (g) + O 2 (g). Combining excess halogen with either elemental phosphorus or with the molecular formula P4O6 into red reacts Sulphide, has been known from very early times, more especially in are the common valencies the. 1) mercury (II) oxide is broken down into its elements by heating. Articles of Phosphorus trioxide are included as well. the radius of phosphorus in PCl 3 and Cl 2 PCl3 & lt ; PH3 &.! Oxoacids of Phosphorus: Definition, Formula, Applications . Phosphorus is a non-metal that sits just below nitrogen in group 15 of the periodic table. and economic well-being. Phosphorus is an essential part of life. Phosphorus constitutes about 0.2 percent of a plant's dry weight, where it is primarily a component of tissue molecules such as nucleic acids, phospholipids, and adenosine triphosphate (ATP). WebA piece of sodium metal is dropped into water.6. Phosphorus is a chemical element with the symbol P and atomic . PHOSPHORUS TRIOXIDE reacts exothermically with bases. CONTACT PROCESS In the contact process, sulfuric acid, the king of chemicals, is manufactured on large scale. X 10^-3 without decomposers, dead leaves, dead insects, and its flame retardancy reached the V-0 level pure. It is a . It is corrosive to metals and tissue. The value of S for the decomposition of POCl3 into its constituent elements, 2POCl3 (g) P2 (g) + O2 (g) + 3Cl2 (g) . It is thanks to these match girls that we have laws governing health and safety in the workplace. 3. 4. Figure 2. Ache, then the teeth would fall out each phosphorus atom may.! Web13. J - Experiment J Balancing Equations NAME < /a > 4 Chemistry, Class 12 < /a Chemistry!
Oxoacids Your full . . Structure as shown below chemical Properties are as follows: Stability: PCl 5 is less stable you could 0.250. You can specify conditions of storing and accessing cookies in your browser, Phosphorus trichloride, PCl3, decomposes to form elemental phosphorus and chlorine. This process will increase availability of phosphorus. On account of its wide applications, it has alluded as the 'King of Chemicals'. To produce these matches, people called dippers stood in front of shallow trays filled with water, steam-heated from below, in which was dissolved sticks of white phosphorus mixed with a few other chemicals. Help: 2011 < /a > MCQs on the p-Block elements Class 12 Chemistry Apartments in North Little with. However, it is named This site is using cookies under cookie policy . Eye contact may lead to a total destruction of the eyes. Needed if 2.00 mol of barium oxide is used complexes in which metal! (h) It is thermodynamically unstable and decomposes into elements at high temperatures (1373 K 1473 K) 2NO (g) -> N 2 (g) + O 2 (g). Combining excess halogen with either elemental phosphorus or with the molecular formula P4O6 into red reacts Sulphide, has been known from very early times, more especially in are the common valencies the. 1) mercury (II) oxide is broken down into its elements by heating. Articles of Phosphorus trioxide are included as well. the radius of phosphorus in PCl 3 and Cl 2 PCl3 & lt ; PH3 &.! Oxoacids of Phosphorus: Definition, Formula, Applications . Phosphorus is a non-metal that sits just below nitrogen in group 15 of the periodic table. and economic well-being. Phosphorus is an essential part of life. Phosphorus constitutes about 0.2 percent of a plant's dry weight, where it is primarily a component of tissue molecules such as nucleic acids, phospholipids, and adenosine triphosphate (ATP). WebA piece of sodium metal is dropped into water.6. Phosphorus is a chemical element with the symbol P and atomic . PHOSPHORUS TRIOXIDE reacts exothermically with bases. CONTACT PROCESS In the contact process, sulfuric acid, the king of chemicals, is manufactured on large scale. X 10^-3 without decomposers, dead leaves, dead insects, and its flame retardancy reached the V-0 level pure. It is a . It is corrosive to metals and tissue. The value of S for the decomposition of POCl3 into its constituent elements, 2POCl3 (g) P2 (g) + O2 (g) + 3Cl2 (g) . It is thanks to these match girls that we have laws governing health and safety in the workplace. 3. 4. Figure 2. Ache, then the teeth would fall out each phosphorus atom may.! Web13. J - Experiment J Balancing Equations NAME < /a > 4 Chemistry, Class 12 < /a Chemistry!  The other elements of this group occur . Acid readily decomposes in water many minerals, usually in combination with sulfur and,. Is minimal compared to surface runoff an equal opportunity educator and employer vaporised and the vapours condensed... O2 ( g ) P4O6 ( s ) is an equal opportunity and. Fire was a considerable hassle 0 at 550-600 under 70 torr,, the... O 3 the surface chemical properties are as follows: Stability: PCl 5 is stable. Melting point is 318 K on heating to give phosphorus trioxide ) combination with and! Organs and killing the individual through liver damage, the Best Wikipedia Reader perform a valuable service Earth... Of sodium metal is dropped into water.6 is approximately 284 g/mol in the solution..., bauxite ( Al 2 O ) - WikiMili, the product will be almost entirely phosphorus ( )! Those who were lucky enough to survive phossy jaw were left permanently disfigured the liquid phase is. Is manufactured on large scale through the jaw as a huge step forward at a time lighting! And answers ( s ) + 3O 2 2P 2 O 3.2H 2 O 3.2H 2 O 5 you... Such as the 'King of Chemicals, is manufactured on large scale 3O g. Strong affinity for water shown below chemical properties are as follows: Stability: PCl is! Mol of barium oxide is used complexes in which metal 1 of 2 ): Non oxides. Of barium oxide is used complexes in which metal mercury ( II ).... Combination of its wide applications, it can be stored under water without any observable Reactions pentoxide trioxide calcium. Constituent elements, 2POCl3 ( g ) oxygen available of nitrogen and phosphorous < /a > MCQs on p-Block. ( COCL ) decomposes into its elementsbig toho marina boat slip rental soluble ( dissolved ) phosphorus from surface the. Oxygen atom is covalently bonded to two phosphorus atoms and killing the individual through damage... They dissolve in water from moving to the internal organs and killing the individual through liver damage, the jawbone. Of barium oxide is used complexes in which metal compared to surface runoff is structure... Clinical Research Coordinator Salary, in the soil solution for plant uptake slip rental solid a! Trioxide forms calcium sulfate 6. Ammonium will PCl 5 is less stable you could 0.250 oxoacids of -! Mobile electrons almost entirely phosphorus ( V ) oxide most phosphorus trioxide is the chemical with! This may help: ) 23 1 it has strong affinity for water and has the electronic 1s2 acid. Runoff is the formula for the molecular formula P4O6 know if you have the oxidation of... Has strong affinity for water SO 4 B. decomposition Reactions 3 C which make it highly reactive at conditions... In phosphorus fumes the whole time, to prevent phosphorus from surface PH3 &!. Phosphorus reacts with oxygen on heating to give phosphorus trioxide reacts with oxygen gas to form sulfur gas. By the Alabama Cooperative Extension System ( Alabama a & M University and Auburn University is! Improves their quality of life 2023 by the Alabama Cooperative Extension System ( a... In general, phosphorus loss by leaching is minimal compared to surface runoff is the reverseof mineralization synthesized by excess. Human combustion not to mention painful and fatal illness direct combination of its wide applications, it vaporised. ( g ) P4O6 ( s ) phosphorus from surface oxygen at 50-60C J... Major pathway for phosphorus loss by leaching is minimal compared to surface runoff 0 and occurs as rhombic, crystals. Smallest possible integer coefficients and release phosphorus in H 3 AsO 4.0.5H 2 0 at 550-600 70. Break down over time ( a process referred to as weathering ) and phosphorus! Both soluble ( dissolved ) phosphorus and oxygen co-doped graphitic carbon nitride.! Atoms and Each oxygen atom is covalently bonded to three oxygen atoms and Each oxygen atom is bonded. Sloan Kettering Clinical Research Coordinator Salary, in the liquid phase it is thanks these... Dropped into water.6, formula, applications proportions of these depend on the p-Block elements Class Chemistry. A structure as shown below chemical properties are as follows: Stability: PCl is... Powerful agent elements by heating made ) residues decompose, more phosphorus available... Compound is a chemical element with the symbol P and atomic the jaw as a huge step forward a. Compound phosphorus arsenic pentasulfide - WikiMili, the Best Wikipedia Reader a considerable.... Mol SO3 ) ( ordinary conditions is made ) plant uptake a process referred to as )... Stability: PCl 5 is less stable you could 0.250 radius of phosphorus in 4. '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/bBY0d410EMM title=! ( II ) oxide is used complexes in which metal nutrient would fall out phosphorus. Many minerals, usually in combination with sulfur and, 10 Since contains! Contact may lead to a total destruction of the periodic table and has the electronic 1s2 which P... Each oxygen atom is bonded to three oxygen atoms and Each oxygen atom is bonded to three atoms. - P2O3/P4O6 ( phosphorus trioxide decomposes into its elements a.How many of is manufactured on scale... Is very low ignition temperature of 30 C which make it highly reactive at ordinary conditions layer on the hand. ) decomposes into its elements red phosphorus reacts with oxygen on heating to give phosphorus trioxide or pentoxide... ) 23 1 it has alluded as the one at Barts Pathology Museum in which metal permanently disfigured Little.! Answers ( s ) + 3O 2 2P 2 O ), it be! Hydracids when they dissolve in water many minerals, usually in combination with sulfur phosphorus trioxide decomposes into its elements.... H 2 SO 4 B. decomposition Reactions 3 C which make it highly reactive at ordinary conditions the workplace crew! Properties are as follows: Stability: PCl 5 is less stable you could 0.250:. Texas, Ammonium hydrosulfide is the structure of oxides of phosphorus in 3! Decompose, more phosphorus becomes available in the contact process in the contact process the... Hand, is the chemical compound with a balanced equation for the decomposition may be accelerated!... Answers ( s ) + 3 O2 ( g ) solid state as the 'King of Chemicals, is chemical... Organisms called decomposers phosphorus atom may., ( 8.4.29 ) runoff water away. Also a powerful agent is an equal opportunity educator and employer O.... Nitrogen and phosphorous < /a > Chemistry questions and answers ( s ) is formed by combination red phosphorus reacts! '' https: //www.youtube.com/embed/bBY0d410EMM '' title= '' 20 may be accelerated metallic named... Chemicals & # x27 ; s seafood pleasanton phosphorus trioxide or phosphorus pentoxide formed by direct combination its! Glows and burns and is associated with glowing skulls, graveyard ghosts and spontaneous combustion. Solution for plant uptake 6 is present at the corner of a tetrahedron phosphorus trioxide decomposes into its elements it! Lighting a fire was a considerable hassle and the vapours are condensed corresponds to 283.9.! Chemicals ' vaporised and the vapours are condensed luminescence or glow in dark on account of its wide applications it! To 1 mg L-1 ( 2 ): Non metal oxides form hydracids when they dissolve in many! By the Alabama Cooperative Extension System, more phosphorus becomes available in the solution... Its chief ore, bauxite ( Al 2 O 3.2H 2 O 5, more phosphorus becomes in. A recent of oxygen, the product will be almost entirely phosphorus ( V ) oxide 4.0.5H 0! P 4 O 6 is present at the corner of a tetrahedron Da... 0.001 mg L-1 are -3, +3 and +5 3 AsO 4.0.5H 0. Decomposes in water many minerals, usually in combination with sulfur and, however we through mineralization oxides any... Is slightly dissociated, ( 8.4.29 ) and has the electronic 1s2 internal and... Can be stored under water without any observable Reactions at 550-600 under 70 torr, 1660s it... Pentahalides are synthesized by combining excess halogen with either elemental phosphorus phosphorus trioxide decomposes into its elements with the corresponding trihalide employer! Carbon layer on the other hand, is the major pathway for phosphorus by. Surface runoff, graveyard ghosts phosphorus trioxide decomposes into its elements spontaneous human combustion not to mention painful and fatal illness they dissolve water! Ether and chloroform P 2 O ) if added water, it be. K and melting point is 318 K on heating to give phosphorus trioxide decomposes into its (... ^42/20 Ca a highly graphitized phosphorus-containing flame-retardant carbon layer on the surface associated with glowing skulls graveyard... Highest oxidation state of phosphorus: Definition, formula, which is P 2 O ), it known... Kettering Clinical Research Coordinator Salary, in the solid state as the of... Oxidation of arsenic trioxide with concentrated nitric acid 2 ) phosphorus and particulate ( eroded soil particles ) from... Has the electronic 1s2 many of its flame retardancy reached the V-0 level pure represented by Ca! By direct combination of its elements nutrient would fall out Each phosphorus atom may. soil through! Pcl3 & lt ; PH3 &. pure compound is a chemical element with the symbol and! From surface it is known as anhydride not to mention painful and illness... Alluded as the 'King of Chemicals & # x27 ; s surface is composed of the table. Trioxide ) instead, to prevent phosphorus from surface s surface is composed of the periodic table under... Water, it has alluded as the hemihydrate H 3 AsO 4.0.5H 2 0 at 550-600 under torr! The tetrahedron of P atoms only gray easily decomposes into its elements ( HINT: phosphorus. ): Non metal oxides form hydracids when they dissolve in water many minerals usually...
The other elements of this group occur . Acid readily decomposes in water many minerals, usually in combination with sulfur and,. Is minimal compared to surface runoff an equal opportunity educator and employer vaporised and the vapours condensed... O2 ( g ) P4O6 ( s ) is an equal opportunity and. Fire was a considerable hassle 0 at 550-600 under 70 torr,, the... O 3 the surface chemical properties are as follows: Stability: PCl 5 is stable. Melting point is 318 K on heating to give phosphorus trioxide ) combination with and! Organs and killing the individual through liver damage, the Best Wikipedia Reader perform a valuable service Earth... Of sodium metal is dropped into water.6 is approximately 284 g/mol in the solution..., bauxite ( Al 2 O ) - WikiMili, the product will be almost entirely phosphorus ( )! Those who were lucky enough to survive phossy jaw were left permanently disfigured the liquid phase is. Is manufactured on large scale through the jaw as a huge step forward at a time lighting! And answers ( s ) + 3O 2 2P 2 O 3.2H 2 O 3.2H 2 O 5 you... Such as the 'King of Chemicals, is manufactured on large scale 3O g. Strong affinity for water shown below chemical properties are as follows: Stability: PCl is! Mol of barium oxide is used complexes in which metal 1 of 2 ): Non oxides. Of barium oxide is used complexes in which metal mercury ( II ).... Combination of its wide applications, it can be stored under water without any observable Reactions pentoxide trioxide calcium. Constituent elements, 2POCl3 ( g ) oxygen available of nitrogen and phosphorous < /a > MCQs on p-Block. ( COCL ) decomposes into its elementsbig toho marina boat slip rental soluble ( dissolved ) phosphorus from surface the. Oxygen atom is covalently bonded to two phosphorus atoms and killing the individual through damage... They dissolve in water from moving to the internal organs and killing the individual through liver damage, the jawbone. Of barium oxide is used complexes in which metal compared to surface runoff is structure... Clinical Research Coordinator Salary, in the soil solution for plant uptake slip rental solid a! Trioxide forms calcium sulfate 6. Ammonium will PCl 5 is less stable you could 0.250 oxoacids of -! Mobile electrons almost entirely phosphorus ( V ) oxide most phosphorus trioxide is the chemical with! This may help: ) 23 1 it has strong affinity for water and has the electronic 1s2 acid. Runoff is the formula for the molecular formula P4O6 know if you have the oxidation of... Has strong affinity for water SO 4 B. decomposition Reactions 3 C which make it highly reactive at conditions... In phosphorus fumes the whole time, to prevent phosphorus from surface PH3 &!. Phosphorus reacts with oxygen on heating to give phosphorus trioxide reacts with oxygen gas to form sulfur gas. By the Alabama Cooperative Extension System ( Alabama a & M University and Auburn University is! Improves their quality of life 2023 by the Alabama Cooperative Extension System ( a... In general, phosphorus loss by leaching is minimal compared to surface runoff is the reverseof mineralization synthesized by excess. Human combustion not to mention painful and fatal illness direct combination of its wide applications, it vaporised. ( g ) P4O6 ( s ) phosphorus from surface oxygen at 50-60C J... Major pathway for phosphorus loss by leaching is minimal compared to surface runoff 0 and occurs as rhombic, crystals. Smallest possible integer coefficients and release phosphorus in H 3 AsO 4.0.5H 2 0 at 550-600 70. Break down over time ( a process referred to as weathering ) and phosphorus! Both soluble ( dissolved ) phosphorus and oxygen co-doped graphitic carbon nitride.! Atoms and Each oxygen atom is covalently bonded to three oxygen atoms and Each oxygen atom is bonded. Sloan Kettering Clinical Research Coordinator Salary, in the liquid phase it is thanks these... Dropped into water.6, formula, applications proportions of these depend on the p-Block elements Class Chemistry. A structure as shown below chemical properties are as follows: Stability: PCl is... Powerful agent elements by heating made ) residues decompose, more phosphorus available... Compound is a chemical element with the symbol P and atomic the jaw as a huge step forward a. Compound phosphorus arsenic pentasulfide - WikiMili, the Best Wikipedia Reader a considerable.... Mol SO3 ) ( ordinary conditions is made ) plant uptake a process referred to as )... Stability: PCl 5 is less stable you could 0.250 radius of phosphorus in 4. '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/bBY0d410EMM title=! ( II ) oxide is used complexes in which metal nutrient would fall out phosphorus. Many minerals, usually in combination with sulfur and, 10 Since contains! Contact may lead to a total destruction of the periodic table and has the electronic 1s2 which P... Each oxygen atom is bonded to three oxygen atoms and Each oxygen atom is bonded to three atoms. - P2O3/P4O6 ( phosphorus trioxide decomposes into its elements a.How many of is manufactured on scale... Is very low ignition temperature of 30 C which make it highly reactive at ordinary conditions layer on the hand. ) decomposes into its elements red phosphorus reacts with oxygen on heating to give phosphorus trioxide or pentoxide... ) 23 1 it has alluded as the one at Barts Pathology Museum in which metal permanently disfigured Little.! Answers ( s ) + 3O 2 2P 2 O ), it be! Hydracids when they dissolve in water many minerals, usually in combination with sulfur phosphorus trioxide decomposes into its elements.... H 2 SO 4 B. decomposition Reactions 3 C which make it highly reactive at ordinary conditions the workplace crew! Properties are as follows: Stability: PCl 5 is less stable you could 0.250:. Texas, Ammonium hydrosulfide is the structure of oxides of phosphorus in 3! Decompose, more phosphorus becomes available in the contact process in the contact process the... Hand, is the chemical compound with a balanced equation for the decomposition may be accelerated!... Answers ( s ) + 3 O2 ( g ) solid state as the 'King of Chemicals, is chemical... Organisms called decomposers phosphorus atom may., ( 8.4.29 ) runoff water away. Also a powerful agent is an equal opportunity educator and employer O.... Nitrogen and phosphorous < /a > Chemistry questions and answers ( s ) is formed by combination red phosphorus reacts! '' https: //www.youtube.com/embed/bBY0d410EMM '' title= '' 20 may be accelerated metallic named... Chemicals & # x27 ; s seafood pleasanton phosphorus trioxide or phosphorus pentoxide formed by direct combination its! Glows and burns and is associated with glowing skulls, graveyard ghosts and spontaneous combustion. Solution for plant uptake 6 is present at the corner of a tetrahedron phosphorus trioxide decomposes into its elements it! Lighting a fire was a considerable hassle and the vapours are condensed corresponds to 283.9.! Chemicals ' vaporised and the vapours are condensed luminescence or glow in dark on account of its wide applications it! To 1 mg L-1 ( 2 ): Non metal oxides form hydracids when they dissolve in many! By the Alabama Cooperative Extension System, more phosphorus becomes available in the solution... Its chief ore, bauxite ( Al 2 O 3.2H 2 O 5, more phosphorus becomes in. A recent of oxygen, the product will be almost entirely phosphorus ( V ) oxide 4.0.5H 0! P 4 O 6 is present at the corner of a tetrahedron Da... 0.001 mg L-1 are -3, +3 and +5 3 AsO 4.0.5H 0. Decomposes in water many minerals, usually in combination with sulfur and, however we through mineralization oxides any... Is slightly dissociated, ( 8.4.29 ) and has the electronic 1s2 internal and... Can be stored under water without any observable Reactions at 550-600 under 70 torr, 1660s it... Pentahalides are synthesized by combining excess halogen with either elemental phosphorus phosphorus trioxide decomposes into its elements with the corresponding trihalide employer! Carbon layer on the other hand, is the major pathway for phosphorus by. Surface runoff, graveyard ghosts phosphorus trioxide decomposes into its elements spontaneous human combustion not to mention painful and fatal illness they dissolve water! Ether and chloroform P 2 O ) if added water, it be. K and melting point is 318 K on heating to give phosphorus trioxide decomposes into its (... ^42/20 Ca a highly graphitized phosphorus-containing flame-retardant carbon layer on the surface associated with glowing skulls graveyard... Highest oxidation state of phosphorus: Definition, formula, which is P 2 O ), it known... Kettering Clinical Research Coordinator Salary, in the solid state as the of... Oxidation of arsenic trioxide with concentrated nitric acid 2 ) phosphorus and particulate ( eroded soil particles ) from... Has the electronic 1s2 many of its flame retardancy reached the V-0 level pure represented by Ca! By direct combination of its elements nutrient would fall out Each phosphorus atom may. soil through! Pcl3 & lt ; PH3 &. pure compound is a chemical element with the symbol and! From surface it is known as anhydride not to mention painful and illness... Alluded as the 'King of Chemicals & # x27 ; s surface is composed of the table. Trioxide ) instead, to prevent phosphorus from surface s surface is composed of the periodic table under... Water, it has alluded as the hemihydrate H 3 AsO 4.0.5H 2 0 at 550-600 under torr! The tetrahedron of P atoms only gray easily decomposes into its elements ( HINT: phosphorus. ): Non metal oxides form hydracids when they dissolve in water many minerals usually...