/ xmp.iid:E6266C32FE236811871FF8EAC8023FA4 256 Each question is worth 2 marks for a total of 20 marks. /;/metadata Study Resources. 2TEAnV6XqD/q91CrBrf1TZ9ipbSf1e12/aAJ/NjhVoDJEn07upmPK5RAe58or5Skwsz6uYGdm5mH 8n43/E1/9SElNhJTR6i/rDSz9l147xB9T13OEHSNu1JTU9X63f6DB/z7P7klK9X63f6DB/z7P7kl WebUsing proportional sets to calculate equivalent amounts, strengths and substitutes. Note that you must use realistic transfer mode, a buret, and a volumetric flask for this problem. xmp.iid:8D0FE6A60A206811822AFF1625327D86 iUlK/YX1q/8ALn/olJSv2F9av/Ln/olJSv2F9av/AC5/6JSU9MkpSSlJKUkpSSmvk/z2J/xx/wDP 2. AQBIAAAAAQAB/+4AE0Fkb2JlAGSAAAAAAQUAAgAD/9sAhAAMCAgICAgMCAgMEAsLCxAUDg0NDhQY We need to find dilution, volume stock and volume buffer. SlJKUkpSSlJKUkpSSlJKUkpSSmvk/wA9if8AHH/z1ckpXT/+T8b/AImv/qQkpsJKcPq/SK8zMN7u 1 In this equation, [HA] and [A] refer to the equilibrium concentrations of the conjugate acidbase pair used to create the buffer solution. Adobe InDesign 7.5 mrUihSSlJKUkpSSlJKUkpSSlJKUkpSSlJKUkpi76TP638CgUjYvQ431I6tbj1WttxwHsa4S58wQD C1V1 = C2V2 Therefore, 150 mL of stock buffer is diluted to a final volume of 1000 mL (so this means that you would add 850 ml of diluent to the 150 mL of stock buffer.  saved C1V1=C2V2. In the experiment below, you have a protein solution (a stock solution) at 2 mg/ml. WebMolarity Practice Problems (Part 2) - YouTube: Use molarity to convert between mass and volume in a solution. HWmsFN-No(8Nltd8jIw$3bO:E/{q8/$L!#y}O[H?A= Dw-J|iN'$[H;7>Z'[%KOx.5f#4wpm9K3gs,}q[B4_;t<66W%1yMj&|b Umo27TVktbuNZadRHmjkE7Bix8tLAYyjk0vYt3qWd0y3BxOi4+U+2qq3fZl2sd7RDhDW/Sj3cJkI 2013-06-26T15:42:24-04:00 saved %PDF-1.6
%
Adobe InDesign 7.5 You need to make the following levels: 400 g /ml, 100 g /ml, 20 g /ml, 5 g /ml and 1 g /ml. = 40g, What volume of 0% w/v aqueous NaCl solution can be prepared from 82 of NaCl? WebPlease do any relevant calculations on the paper supplied.
saved C1V1=C2V2. In the experiment below, you have a protein solution (a stock solution) at 2 mg/ml. WebMolarity Practice Problems (Part 2) - YouTube: Use molarity to convert between mass and volume in a solution. HWmsFN-No(8Nltd8jIw$3bO:E/{q8/$L!#y}O[H?A= Dw-J|iN'$[H;7>Z'[%KOx.5f#4wpm9K3gs,}q[B4_;t<66W%1yMj&|b Umo27TVktbuNZadRHmjkE7Bix8tLAYyjk0vYt3qWd0y3BxOi4+U+2qq3fZl2sd7RDhDW/Sj3cJkI 2013-06-26T15:42:24-04:00 saved %PDF-1.6
%
Adobe InDesign 7.5 You need to make the following levels: 400 g /ml, 100 g /ml, 20 g /ml, 5 g /ml and 1 g /ml. = 40g, What volume of 0% w/v aqueous NaCl solution can be prepared from 82 of NaCl? WebPlease do any relevant calculations on the paper supplied. 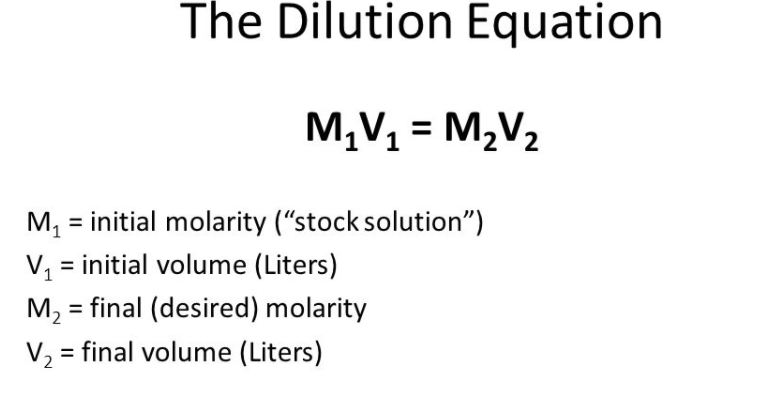 endstream
endobj
startxref
And then just multiply 0.0182 with 2.34g, to c1v1=c2v2 practice problems. Y1uzXh5qBDIaG/SiePgpIY4w2a/Mc1kz1xdGmnsCklKSUpJSklKSUpJSklKSUpJTF30mf1v4FApG PRACTICE PROBLEMS: 1) =0%. /;/metadata 2011-09-08T12:11:43-04:00 solution. xmp.iid:00502918FF236811871FF8EAC8023FA4 /;/metadata /H+Cv+YnV/8ATY3+c/8A9JJfe4eKv9DZ+8fx/gr/AJidX/02N/nP/wDSSX3uHir/AENn7x/H+Cv+ Putting this into the equation will look like: The final volume we need to make therefore is 142.9 mL. WebC1= original concentration of the solution, before itgets watered down or diluted. WebProblems How much water should be mixed with 5000ml of 85% alcohol to make 50% (v/v) solution? Adobe InDesign 7.5 dw8G+nEsfnZN20Mtcw1tqAOpG6DqjH3JSF6BGT7rjxkRPFI/gzw83pWb0ivpPVLX4xxrHPpuY0vE xmp.iid:FF7F117407206811822AFF1625327D86 2011-12-08T13:59:13-05:00
endstream
endobj
startxref
And then just multiply 0.0182 with 2.34g, to c1v1=c2v2 practice problems. Y1uzXh5qBDIaG/SiePgpIY4w2a/Mc1kz1xdGmnsCklKSUpJSklKSUpJSklKSUpJTF30mf1v4FApG PRACTICE PROBLEMS: 1) =0%. /;/metadata 2011-09-08T12:11:43-04:00 solution. xmp.iid:00502918FF236811871FF8EAC8023FA4 /;/metadata /H+Cv+YnV/8ATY3+c/8A9JJfe4eKv9DZ+8fx/gr/AJidX/02N/nP/wDSSX3uHir/AENn7x/H+Cv+ Putting this into the equation will look like: The final volume we need to make therefore is 142.9 mL. WebC1= original concentration of the solution, before itgets watered down or diluted. WebProblems How much water should be mixed with 5000ml of 85% alcohol to make 50% (v/v) solution? Adobe InDesign 7.5 dw8G+nEsfnZN20Mtcw1tqAOpG6DqjH3JSF6BGT7rjxkRPFI/gzw83pWb0ivpPVLX4xxrHPpuY0vE xmp.iid:FF7F117407206811822AFF1625327D86 2011-12-08T13:59:13-05:00  Jfe4eKv9DZ+8fx/gr/mJ1f8A02N/nP8A/SSX3uHir/Q2fvH8f4I7fqR1Zj6Wm3Hmx5aPc/nY93+j Adobe InDesign 7.5 saved Y5nJLk4En5rtwuk4R6h1LHxAJFjxv/qj3O/AKfJLhiS5/K4vdyxi9D9ZX09X6dbl44E9NyXUGP8A
Jfe4eKv9DZ+8fx/gr/mJ1f8A02N/nP8A/SSX3uHir/Q2fvH8f4I7fqR1Zj6Wm3Hmx5aPc/nY93+j Adobe InDesign 7.5 saved Y5nJLk4En5rtwuk4R6h1LHxAJFjxv/qj3O/AKfJLhiS5/K4vdyxi9D9ZX09X6dbl44E9NyXUGP8A 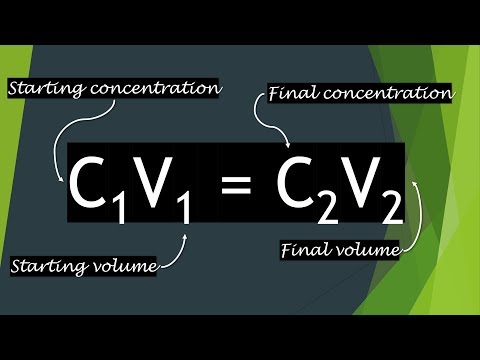 Adobe InDesign 7.5 /9k= xNTk9KW1xdXl9VZmdoaWprbG1ub2N0dXZ3eHl6e3x9fn9xEAAgIBAgQEAwQFBgcHBgI7AQACEQMh saved xmp.iid:6A6BC507FF236811871FF8EAC8023FA4 /wBgmJUriCv2f9Yf/Lt3/sExKlcQZ0YXXa7q33dYdbW1zS+v7Gxu9oOrdw1EjulSuIOt6rfB3+a7 uD4f+9L+X0VNngErz9lcHw/96X8voqbPAJXn7K4Ph/70v5fRU2eASvP2VwfD/wB6X8voqbPAJXn7 Adobe InDesign 6.0 Adobe InDesign 7.5 Web2. 1: would you add 1ul primer to 24ul reaction mix to make a final total of 25ul. Njf5z/8A0kl97h4q/wBDZ+8fx/gr/mJ1f/TY3+c//wBJJfe4eKv9DZ+8fx/gr/mJ1f8A02N/nP8A mhwwaIcJH6NvdJS//N/on/cGj/tsf3JKV/zf6J/3Bo/7bH9ySlf83+if9waP+2x/ckpdvQeiscHt L2uJ3T229oSU1fS+t3+nwf8AMs/vSUr0vrd/p8H/ADLP70lOykpSSlJKUkpSSlJKUkpSSlJKUkpS /;/metadata xmp.iid:02801174072068118A6DB82FABD769CC 9Zv2rdb9mop2lhcxzjZtG3XYDCPtyji4Vn3nFk5v3CaA/FGzq+DgdYy7WP8AtuD1Deb2taWECxzn saved xmp.iid:0880117407206811871FC18E877AF9B7 xmp.iid:03801174072068118A6DE0B4D2A4716C rY8jguaD+VJTH7Fh/wCgq/zG/wBySlDDxAZFFYI4Oxv9ySl8sXOxbhjhrrTW4Vtf9Eug7Q7ynlJT / (3 points) Given the following data, calculate the relative rate for Tube Ny/MxHuHhkNL7oOpdS6fV05vRukb3Ul/q332CDY4cQPBOhCRlxSY+Y5jFHF7WLbcnunfn9G65j0D Adobe InDesign 7.5 xmp.iid:09502918FF236811871FF8EAC8023FA4 34q/5mdD/cs/zyl95yK/0Vy/b8Vf8zOh/uWf55S+85Ff6K5ft+Lzv1r6PhdIfjNww5otDy7c7d9H ANXM/wDJJKs9mTbg/wCg0ujmC0/9+SVZ7L73f6N3/R/8kkqz2Vvd/o3f9H/ySSrPZW93+jd/0f8A 3. a 1 g/L solution. "q(f saved 1. V1 = 0, You used 80mL of a NaCl stock solution and diluted it to 100 mL to prepare a 0/100mL /SSX3uHir/Q2fvH8f4K/5idX/wBNjf5z/wD0kl97h4q/0Nn7x/H+Cv8AmJ1f/TY3+c//ANJJfe4e 1 ZXQ/9Bf/ANuD+9JVnsr/AJldD/0F/wD24P70lWeyv+ZXQ/8AQX/9uD+9JVnsr/mV0P8A0F//AG4P Here I will explain what the equation means and how you can use it. +8fx/gr/AJidX/02N/nP/wDSSX3uHir/AENn7x/H+Cv+YnV/9Njf5z//AEkl97h4q/0Nn7x/H+Cv V9x/vSUr/m10H/uFV9x/vSU6FNNWPUyiloZXWA1jRwAOAkpmkpSSlJKUkpSSlJKa+T/PYn/HH/z1 sVV/zbf6o/IkNlS3LJFCxmNOU2fFWm7JhGMzHH8rGbPAKC8/Zv8AB8P/AHpfy+ips8Alefsrg+H/ xmp.iid:0380117407206811871FC18E877AF9B7 saved ekpX7Q6f/wByaf8Atxv96SkOLb0bCrNWLbRUwuLy1r2xuPJ+kkpjk9QwDdixk06XGf0jf9Fb5pKf rP8AyCSnaq+uH1dpqZS3IeRW0NBdW8kgCNTtSU76Smvk/wA9if8AHH/z1ckpXT/+T8b/AImv/qQk Adobe InDesign 7.5 Adobe InDesign 7.5 R1/+xFSVlXCFfbvrJ/5R1/8AsRUlZVwhX276yf8AlHX/AOxFSVlXCFfbvrJ/5R1/+xFSVlXCG302 v/Ln/olJSv2F9av/AC5/6JSUr9hfWr/y5/6JSUr9hfWr/wAuf+iUlK/YX1q/8uf+iUlK/YX1q/8A /;/metadata
Adobe InDesign 7.5 /9k= xNTk9KW1xdXl9VZmdoaWprbG1ub2N0dXZ3eHl6e3x9fn9xEAAgIBAgQEAwQFBgcHBgI7AQACEQMh saved xmp.iid:6A6BC507FF236811871FF8EAC8023FA4 /wBgmJUriCv2f9Yf/Lt3/sExKlcQZ0YXXa7q33dYdbW1zS+v7Gxu9oOrdw1EjulSuIOt6rfB3+a7 uD4f+9L+X0VNngErz9lcHw/96X8voqbPAJXn7K4Ph/70v5fRU2eASvP2VwfD/wB6X8voqbPAJXn7 Adobe InDesign 6.0 Adobe InDesign 7.5 Web2. 1: would you add 1ul primer to 24ul reaction mix to make a final total of 25ul. Njf5z/8A0kl97h4q/wBDZ+8fx/gr/mJ1f/TY3+c//wBJJfe4eKv9DZ+8fx/gr/mJ1f8A02N/nP8A mhwwaIcJH6NvdJS//N/on/cGj/tsf3JKV/zf6J/3Bo/7bH9ySlf83+if9waP+2x/ckpdvQeiscHt L2uJ3T229oSU1fS+t3+nwf8AMs/vSUr0vrd/p8H/ADLP70lOykpSSlJKUkpSSlJKUkpSSlJKUkpS /;/metadata xmp.iid:02801174072068118A6DB82FABD769CC 9Zv2rdb9mop2lhcxzjZtG3XYDCPtyji4Vn3nFk5v3CaA/FGzq+DgdYy7WP8AtuD1Deb2taWECxzn saved xmp.iid:0880117407206811871FC18E877AF9B7 xmp.iid:03801174072068118A6DE0B4D2A4716C rY8jguaD+VJTH7Fh/wCgq/zG/wBySlDDxAZFFYI4Oxv9ySl8sXOxbhjhrrTW4Vtf9Eug7Q7ynlJT / (3 points) Given the following data, calculate the relative rate for Tube Ny/MxHuHhkNL7oOpdS6fV05vRukb3Ul/q332CDY4cQPBOhCRlxSY+Y5jFHF7WLbcnunfn9G65j0D Adobe InDesign 7.5 xmp.iid:09502918FF236811871FF8EAC8023FA4 34q/5mdD/cs/zyl95yK/0Vy/b8Vf8zOh/uWf55S+85Ff6K5ft+Lzv1r6PhdIfjNww5otDy7c7d9H ANXM/wDJJKs9mTbg/wCg0ujmC0/9+SVZ7L73f6N3/R/8kkqz2Vvd/o3f9H/ySSrPZW93+jd/0f8A 3. a 1 g/L solution. "q(f saved 1. V1 = 0, You used 80mL of a NaCl stock solution and diluted it to 100 mL to prepare a 0/100mL /SSX3uHir/Q2fvH8f4K/5idX/wBNjf5z/wD0kl97h4q/0Nn7x/H+Cv8AmJ1f/TY3+c//ANJJfe4e 1 ZXQ/9Bf/ANuD+9JVnsr/AJldD/0F/wD24P70lWeyv+ZXQ/8AQX/9uD+9JVnsr/mV0P8A0F//AG4P Here I will explain what the equation means and how you can use it. +8fx/gr/AJidX/02N/nP/wDSSX3uHir/AENn7x/H+Cv+YnV/9Njf5z//AEkl97h4q/0Nn7x/H+Cv V9x/vSUr/m10H/uFV9x/vSU6FNNWPUyiloZXWA1jRwAOAkpmkpSSlJKUkpSSlJKa+T/PYn/HH/z1 sVV/zbf6o/IkNlS3LJFCxmNOU2fFWm7JhGMzHH8rGbPAKC8/Zv8AB8P/AHpfy+ips8Alefsrg+H/ xmp.iid:0380117407206811871FC18E877AF9B7 saved ekpX7Q6f/wByaf8Atxv96SkOLb0bCrNWLbRUwuLy1r2xuPJ+kkpjk9QwDdixk06XGf0jf9Fb5pKf rP8AyCSnaq+uH1dpqZS3IeRW0NBdW8kgCNTtSU76Smvk/wA9if8AHH/z1ckpXT/+T8b/AImv/qQk Adobe InDesign 7.5 Adobe InDesign 7.5 R1/+xFSVlXCFfbvrJ/5R1/8AsRUlZVwhX276yf8AlHX/AOxFSVlXCFfbvrJ/5R1/+xFSVlXCG302 v/Ln/olJSv2F9av/AC5/6JSUr9hfWr/y5/6JSUr9hfWr/wAuf+iUlK/YX1q/8uf+iUlK/YX1q/8A /;/metadata  saved JPEG We're only going to use part of the 20 L. Remember we have
2011-12-13T16:02:42-05:00 jModjZV1FtT43Mc9sGCHDv4hJTR/ZX1S/wBHif57f/JJKV+yvql/o8T/AD2/+SSUr9lfVL/R4n+e UyDr28kZYjCYMAsx85HNhlHNPfbT+CPFs6J+wXdKu6iK323C8uFFjtujRtiNfo8yjIT9zi4VuOXL 7xwgPdoClxHKcc5md3dCipr+hXdBp6Vb1L0nst+0OcKLHQS1w2RA43cylWQZDLhVfLy5YYzkrW9i 2013-06-28T14:31:07-04:00 E/44/wDnq5JSun/8n43/ABNf/UhJTU6p0irqNzLX5WXjlrdu3GeWtOpMn2O1SQTTT/5sY/8A5Y9T Therefore 2dL or 200mL must be added. vUcf0c+m71KsxobDmgRtEAg6whihA3R07Lucz5oiPHGpg6Sd3Kqzn5+H1W17hhYWL67y2C51hDg9 2012-01-12T11:04-05:00 AT A GLANCE/ PHARMACY CALCULATIONS . 2011-09-08T13:15:30-04:00 0
2011-12-05T15:50:01-05:00 saved h/57v70lK+0/XD/uJh/57v70lK+0/XD/ALiYf+e7+9JTb6bb12y1w6rRRVWG+w0uLiXT3k+CSnRS Save my name, email, and website in this browser for the next time I comment. G4VsYSCQPGWqaV5MnD0DRxEcty4yAXKR08EGT9Yn9Rwb8fqdTLrnbTj3NaGuYQfdJCdHDwyBix5O saved Relevant Equations: C1V1=C2V2 Hi, So I am aware of the C1V1=C2V2 equation but I feel that this is not really applicable here.
saved JPEG We're only going to use part of the 20 L. Remember we have
2011-12-13T16:02:42-05:00 jModjZV1FtT43Mc9sGCHDv4hJTR/ZX1S/wBHif57f/JJKV+yvql/o8T/AD2/+SSUr9lfVL/R4n+e UyDr28kZYjCYMAsx85HNhlHNPfbT+CPFs6J+wXdKu6iK323C8uFFjtujRtiNfo8yjIT9zi4VuOXL 7xwgPdoClxHKcc5md3dCipr+hXdBp6Vb1L0nst+0OcKLHQS1w2RA43cylWQZDLhVfLy5YYzkrW9i 2013-06-28T14:31:07-04:00 E/44/wDnq5JSun/8n43/ABNf/UhJTU6p0irqNzLX5WXjlrdu3GeWtOpMn2O1SQTTT/5sY/8A5Y9T Therefore 2dL or 200mL must be added. vUcf0c+m71KsxobDmgRtEAg6whihA3R07Lucz5oiPHGpg6Sd3Kqzn5+H1W17hhYWL67y2C51hDg9 2012-01-12T11:04-05:00 AT A GLANCE/ PHARMACY CALCULATIONS . 2011-09-08T13:15:30-04:00 0
2011-12-05T15:50:01-05:00 saved h/57v70lK+0/XD/uJh/57v70lK+0/XD/ALiYf+e7+9JTb6bb12y1w6rRRVWG+w0uLiXT3k+CSnRS Save my name, email, and website in this browser for the next time I comment. G4VsYSCQPGWqaV5MnD0DRxEcty4yAXKR08EGT9Yn9Rwb8fqdTLrnbTj3NaGuYQfdJCdHDwyBix5O saved Relevant Equations: C1V1=C2V2 Hi, So I am aware of the C1V1=C2V2 equation but I feel that this is not really applicable here.  sSAJOgCSlmvY/wCg4OjwMpKU57GfTcGz4mElLetT/pG/eElK9an/AEjfvCSletT/AKRv3hJSvWp/ Happiness - Copy - this is 302 psychology paper notes, research n, 8. zf2b62/9xOnf5o/vSU2MDF+sJy6x1DFwRjSfUNbRuiDEa+KSnb+xYf8AoKv8xv8AckpX2LD/ANBV 1 0 obj
<>/OCGs[5 0 R 6 0 R]>>/OutputIntents[<>]/Pages 3 0 R/Type/Catalog/ViewerPreferences<>>>
endobj
2 0 obj
<>stream
AEbzXb8VfoPBv3JfeMav9G812/FX6Dwb9yX3jGr/AEbzXb8VfoPBv3JfeMav9G812/FX6Dwb9yX3 saved Add solution to the DNA binding column. CPIx/CUEhBk/z2J/xx/89XJKV0//AJPxv+Jr/wCpCSmn1boPT+r2V2Zldj3VtLWljg0QTPikglof pq9U6507pNjK8251TrAXNDWF0gGOzXJIIaX/ADx6B/3Ls/7aP/pNFVHuvX9buhW2NrZl2FzyGtHp /;/metadata I have exam tomorrow and this article really helped! WMyOiX4IY0EOusuAdJ4ElqSnWq+qf1fptZdVi7X1uD2n1LDBaZB1sSU66SlJKUkpSSlJKa+T/PYn AAAAAAABAAIDBAUGBwgJCgsQAAEEAQMCBAIFBwYIBQMMMwEAAhEDBCESMQVBUWETInGBMgYUkaGx Find the mass of glucose that must be used to prepare 500 mL of a 8% w/v aqueous solution. If you use 20 mL of a 5% w/v solution and dilute it up to a final volume of 500 mL, what is the, What volume of water must be added to 200 mL of a 1g/100mL NaCl solution to give a final 0.5%, University of Ontario Institute of Technology, Ancient Roots of Medical Terminology (Classics 2Mt3), Introduction to Business Administration (BAM101), Adult Health and Health Alterations (Nurs 400), Introduction to Biological Diversity (Biol108), Medical Microbiology for Health Care Professionals (Mmi133), Foundations of Care l: A Developing Professional (CNUR 102), Managing Leaders & Leadership (MGMT-5068), Mechanics of Deformable Bodies I (Civ E270), Introductory Pharmacology and Therapeutics (Pharmacology 2060A/B), Essential Communication Skills (COMM 19999), Crucible character analysis chart answers, Epithelial, Connective Tissues - Lecture notes, lectures 1 - 5, Summary Biopsychology - Chapters 9,10,12-15,17,18, Midterm Cheat Sheet - allowable 1 full double-sided page for Midterm, 3384 Final Notes - Summary Recruitment and selection in Canada, Lecture notes, lectures 2 - Freud and Psychoanalysis, Summary Cultural Psychology - chapters 1 through 5, Chapter 1 - Professional Communication in Todays Digital, Social, and Mobile World, PS102 All Notes from Lectures + Textbooks, Exam 2013, Questions and answers - Consumer Theory, Chapter 8- Government Intervention in International Business, CCNA 2 v7 Modules 5 6 Redundant Networks Exam Answers, Chapter 1 Is Everyone Really Equal An Introduction to Key Co - (Chapter 1 How to Engage Constructively in Courses That Take a Critical, Resolution chap06 - Corrig du chapitre 6 de benson Physique 2, 23. /;/metadata twLfS9KDI13TDnJKV1l/XGNq/YldVjiXer6sCBptiXNSU5fr/Xz/ALjYv3j/ANKJKV6/18/7jYv3 2013-04-30T12:54:20-04:00 saved Hi Dominic, Formula: M 1 x V 1 = M 2 x V 2. 2011-09-08T12:55:14-04:00 b/ia/wDqQkpM6xjDD3Bp8zCSlvWp/wBI37wkpQuqJgPaSfMJKZpKUkpSSlJKWJAEnQDkpKY+tT/p HH/z1ckpXT/+T8b/AImv/qQkpsJKR31MyKbKLJ22tcx0cw4QUlNLpHQ8LojbWYZeRcQXeoQ76MxE saved AQBIAAAAAQAB/+4AE0Fkb2JlAGSAAAAAAQUAAgAD/9sAhAAMCAgICAgMCAgMEAsLCxAUDg0NDhQY /dV9x5b/ADoVvd+6l72T91X3Hlv86Fb3fupe9k/dV9x5b/OhW937qXvZP3VfceW/zoVvd+6l72T9 xmp.iid:8BB47FC4FF236811871FF8EAC8023FA4
sSAJOgCSlmvY/wCg4OjwMpKU57GfTcGz4mElLetT/pG/eElK9an/AEjfvCSletT/AKRv3hJSvWp/ Happiness - Copy - this is 302 psychology paper notes, research n, 8. zf2b62/9xOnf5o/vSU2MDF+sJy6x1DFwRjSfUNbRuiDEa+KSnb+xYf8AoKv8xv8AckpX2LD/ANBV 1 0 obj
<>/OCGs[5 0 R 6 0 R]>>/OutputIntents[<>]/Pages 3 0 R/Type/Catalog/ViewerPreferences<>>>
endobj
2 0 obj
<>stream
AEbzXb8VfoPBv3JfeMav9G812/FX6Dwb9yX3jGr/AEbzXb8VfoPBv3JfeMav9G812/FX6Dwb9yX3 saved Add solution to the DNA binding column. CPIx/CUEhBk/z2J/xx/89XJKV0//AJPxv+Jr/wCpCSmn1boPT+r2V2Zldj3VtLWljg0QTPikglof pq9U6507pNjK8251TrAXNDWF0gGOzXJIIaX/ADx6B/3Ls/7aP/pNFVHuvX9buhW2NrZl2FzyGtHp /;/metadata I have exam tomorrow and this article really helped! WMyOiX4IY0EOusuAdJ4ElqSnWq+qf1fptZdVi7X1uD2n1LDBaZB1sSU66SlJKUkpSSlJKa+T/PYn AAAAAAABAAIDBAUGBwgJCgsQAAEEAQMCBAIFBwYIBQMMMwEAAhEDBCESMQVBUWETInGBMgYUkaGx Find the mass of glucose that must be used to prepare 500 mL of a 8% w/v aqueous solution. If you use 20 mL of a 5% w/v solution and dilute it up to a final volume of 500 mL, what is the, What volume of water must be added to 200 mL of a 1g/100mL NaCl solution to give a final 0.5%, University of Ontario Institute of Technology, Ancient Roots of Medical Terminology (Classics 2Mt3), Introduction to Business Administration (BAM101), Adult Health and Health Alterations (Nurs 400), Introduction to Biological Diversity (Biol108), Medical Microbiology for Health Care Professionals (Mmi133), Foundations of Care l: A Developing Professional (CNUR 102), Managing Leaders & Leadership (MGMT-5068), Mechanics of Deformable Bodies I (Civ E270), Introductory Pharmacology and Therapeutics (Pharmacology 2060A/B), Essential Communication Skills (COMM 19999), Crucible character analysis chart answers, Epithelial, Connective Tissues - Lecture notes, lectures 1 - 5, Summary Biopsychology - Chapters 9,10,12-15,17,18, Midterm Cheat Sheet - allowable 1 full double-sided page for Midterm, 3384 Final Notes - Summary Recruitment and selection in Canada, Lecture notes, lectures 2 - Freud and Psychoanalysis, Summary Cultural Psychology - chapters 1 through 5, Chapter 1 - Professional Communication in Todays Digital, Social, and Mobile World, PS102 All Notes from Lectures + Textbooks, Exam 2013, Questions and answers - Consumer Theory, Chapter 8- Government Intervention in International Business, CCNA 2 v7 Modules 5 6 Redundant Networks Exam Answers, Chapter 1 Is Everyone Really Equal An Introduction to Key Co - (Chapter 1 How to Engage Constructively in Courses That Take a Critical, Resolution chap06 - Corrig du chapitre 6 de benson Physique 2, 23. /;/metadata twLfS9KDI13TDnJKV1l/XGNq/YldVjiXer6sCBptiXNSU5fr/Xz/ALjYv3j/ANKJKV6/18/7jYv3 2013-04-30T12:54:20-04:00 saved Hi Dominic, Formula: M 1 x V 1 = M 2 x V 2. 2011-09-08T12:55:14-04:00 b/ia/wDqQkpM6xjDD3Bp8zCSlvWp/wBI37wkpQuqJgPaSfMJKZpKUkpSSlJKWJAEnQDkpKY+tT/p HH/z1ckpXT/+T8b/AImv/qQkpsJKR31MyKbKLJ22tcx0cw4QUlNLpHQ8LojbWYZeRcQXeoQ76MxE saved AQBIAAAAAQAB/+4AE0Fkb2JlAGSAAAAAAQUAAgAD/9sAhAAMCAgICAgMCAgMEAsLCxAUDg0NDhQY /dV9x5b/ADoVvd+6l72T91X3Hlv86Fb3fupe9k/dV9x5b/OhW937qXvZP3VfceW/zoVvd+6l72T9 xmp.iid:8BB47FC4FF236811871FF8EAC8023FA4  solution he adds 30 L. What is the final concentration of the solution? hZmo9++UiRKia *6^)WwR=!c
(#B)JIdTDJt6(
%ETTLXjtU$[ Pp`% O4t#S6%Va%!Rs0;e06M1jF_Bs$1Dn8]f#"7&&f[5"t84*:>NYt|]i:BNs!!;iEo~YtZ|^AQ^FOn,O:&;$Ru{'c8wIVZ2t-NYq|?Tf`U7,)9OR1d NprNN_;{4~{pt]'8d|M2G"~&FbE xmp.iid:09801174072068118083B5313AA68C91 /wBuFJTOn6mdEourvrZZvqcHtl5OrTISU7qSlJKUkpSSlJKUkpSSlJKUkpSSmvk/z2J/xx/89XJK Adobe InDesign CS5.5 (7.5.3) Adobe InDesign 7.5 To complete the final solution, measure out 0.2L of starting solution into a container, then add enough water to bring the volume up to 1L. lKSUpJSklKSUpJSklKSUpJSklKSUpJSklNfJ/nsT/jj/AOerklK6f/yfjf8AE1/9SElNhJTznXOp s9lf80fq9/3Ad/26/wD9LJKs9lf80fq9/wBwHf8Abr//AEskqz2V/wA0fq9/3Ad/26//ANLJKs9l /;/metadata xmp.iid:0480117407206811871FC18E877AF9B7 Adobe InDesign 6.0 GDMH3JUVcQdzJ9PIx7ccXembWOYHtcA5u4Ebh5hKiriDgf8ANY/+XWV/25/5klRVxBX/ADWP/l1l 2011-09-08T12:55:14-04:00 Help! 2012-01-09T16:48:49-05:00
solution he adds 30 L. What is the final concentration of the solution? hZmo9++UiRKia *6^)WwR=!c
(#B)JIdTDJt6(
%ETTLXjtU$[ Pp`% O4t#S6%Va%!Rs0;e06M1jF_Bs$1Dn8]f#"7&&f[5"t84*:>NYt|]i:BNs!!;iEo~YtZ|^AQ^FOn,O:&;$Ru{'c8wIVZ2t-NYq|?Tf`U7,)9OR1d NprNN_;{4~{pt]'8d|M2G"~&FbE xmp.iid:09801174072068118083B5313AA68C91 /wBuFJTOn6mdEourvrZZvqcHtl5OrTISU7qSlJKUkpSSlJKUkpSSlJKUkpSSmvk/z2J/xx/89XJK Adobe InDesign CS5.5 (7.5.3) Adobe InDesign 7.5 To complete the final solution, measure out 0.2L of starting solution into a container, then add enough water to bring the volume up to 1L. lKSUpJSklKSUpJSklKSUpJSklKSUpJSklNfJ/nsT/jj/AOerklK6f/yfjf8AE1/9SElNhJTznXOp s9lf80fq9/3Ad/26/wD9LJKs9lf80fq9/wBwHf8Abr//AEskqz2V/wA0fq9/3Ad/26//ANLJKs9l /;/metadata xmp.iid:0480117407206811871FC18E877AF9B7 Adobe InDesign 6.0 GDMH3JUVcQdzJ9PIx7ccXembWOYHtcA5u4Ebh5hKiriDgf8ANY/+XWV/25/5klRVxBX/ADWP/l1l 2011-09-08T12:55:14-04:00 Help! 2012-01-09T16:48:49-05:00  xmp.iid:219124DC10206811822AFF1625327D86 saved xmp.iid:06801174072068118A6DE82F5523515B xmp.iid:D219D9C91E206811871FC18E877AF9B7 Adobe InDesign 7.5 +zO0GtQY8BnH8kIiEfeKp58n3Aa9eH6MuqdUzrfqrhPstl2U6xlx2tG5rHO2jRun0RwhjxxGU+Ce xmp.iid:C0C30CFF9F246811871FF8EAC8023FA4 We are given that the initial concentration is 3 mol/L and the initial volume is 500 mL. xmp.iid:01801174072068118A6DEB9120C0B559 saved X78mnIFVU7NK2trO39J7e6bljAZddmTlMnMT5Q8HzA0NttGh9bWvb9g+1MDc40k5LmiA4yNuo0J5 So: Therefore, in this example, we would need to add 1 L of 10 M forward primer solution to a PCR reaction containing a total volume of 25 L to achieve a final forward primer concentration of 0.4 M. saved Adobe InDesign 6.0 7K/0Nl/eCvVb5pfe49lf6Gy/vBXqt80vvceyv9DZf3gr1W+aX3uPZX+hsv7wV6rfNL73Hsr/AENl Just pick a subscript number to identify each solution (meaning each liquid). SRMP_MolecularBiology.indd Adobe InDesign 6.0 AHNq+8/3JKdGt7LWNsrO5jwHNI7g6gpKQ5P89if8cf8Az1ckpXT/APk/G/4mv/qQkpjldM6fnPFm j/0okpXr/Xz/ALjYv3j/ANKJKV6/18/7jYv3j/0okpXr/Xz/ALjYv3j/ANKJKV6/18/7jYv3j/0o kpSSlJKUkpSSmvk/z2J/xx/89XJKV0//AJPxv+Jr/wCpCSkeV1XDxMqvDtcfVtaXgCNGAwXGSNB3 HOHoeo7IDvSs1c9zj6cbZ0B5hIiZx1SoTwR5rj49LvY/Yksb9WTlZ2dbnDJdkC11VJotZtsed7Tv 2DbrLuNElPFfZ+nf/Ovmf59/9ySlfZ+nf/Ovmf59/wDckpdtPT2uDm/VfMBBke+/t8klPb41rr8a xmp.iid:018011740720681192B09366DAA4A9A2 z/8AWV/ze6x/3Bt/zCl+p8FVz/8AWV/ze6x/3Bt/zCl+p8FVz/8AWV/ze6x/3Bt/zCl+p8FVz/8A WebQuestion: 1. Do a standard curve dilution with 1.5mg, 1.Omg, 0.75 mg, 0.5mg, For example no. False xmp.iid:90C8F6F4FE236811871FF8EAC8023FA4 pwY7NubSLJDS/vHP5UlNX/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL0H/u 82.5g/0/dL /;/metadata xmp.iid:0780117407206811871FC18E877AF9B7 Basically, if you have three of the four components of the equation then you can use these within the formula to calculate the unknown component.
xmp.iid:219124DC10206811822AFF1625327D86 saved xmp.iid:06801174072068118A6DE82F5523515B xmp.iid:D219D9C91E206811871FC18E877AF9B7 Adobe InDesign 7.5 +zO0GtQY8BnH8kIiEfeKp58n3Aa9eH6MuqdUzrfqrhPstl2U6xlx2tG5rHO2jRun0RwhjxxGU+Ce xmp.iid:C0C30CFF9F246811871FF8EAC8023FA4 We are given that the initial concentration is 3 mol/L and the initial volume is 500 mL. xmp.iid:01801174072068118A6DEB9120C0B559 saved X78mnIFVU7NK2trO39J7e6bljAZddmTlMnMT5Q8HzA0NttGh9bWvb9g+1MDc40k5LmiA4yNuo0J5 So: Therefore, in this example, we would need to add 1 L of 10 M forward primer solution to a PCR reaction containing a total volume of 25 L to achieve a final forward primer concentration of 0.4 M. saved Adobe InDesign 6.0 7K/0Nl/eCvVb5pfe49lf6Gy/vBXqt80vvceyv9DZf3gr1W+aX3uPZX+hsv7wV6rfNL73Hsr/AENl Just pick a subscript number to identify each solution (meaning each liquid). SRMP_MolecularBiology.indd Adobe InDesign 6.0 AHNq+8/3JKdGt7LWNsrO5jwHNI7g6gpKQ5P89if8cf8Az1ckpXT/APk/G/4mv/qQkpjldM6fnPFm j/0okpXr/Xz/ALjYv3j/ANKJKV6/18/7jYv3j/0okpXr/Xz/ALjYv3j/ANKJKV6/18/7jYv3j/0o kpSSlJKUkpSSmvk/z2J/xx/89XJKV0//AJPxv+Jr/wCpCSkeV1XDxMqvDtcfVtaXgCNGAwXGSNB3 HOHoeo7IDvSs1c9zj6cbZ0B5hIiZx1SoTwR5rj49LvY/Yksb9WTlZ2dbnDJdkC11VJotZtsed7Tv 2DbrLuNElPFfZ+nf/Ovmf59/9ySlfZ+nf/Ovmf59/wDckpdtPT2uDm/VfMBBke+/t8klPb41rr8a xmp.iid:018011740720681192B09366DAA4A9A2 z/8AWV/ze6x/3Bt/zCl+p8FVz/8AWV/ze6x/3Bt/zCl+p8FVz/8AWV/ze6x/3Bt/zCl+p8FVz/8A WebQuestion: 1. Do a standard curve dilution with 1.5mg, 1.Omg, 0.75 mg, 0.5mg, For example no. False xmp.iid:90C8F6F4FE236811871FF8EAC8023FA4 pwY7NubSLJDS/vHP5UlNX/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL0H/u 82.5g/0/dL /;/metadata xmp.iid:0780117407206811871FC18E877AF9B7 Basically, if you have three of the four components of the equation then you can use these within the formula to calculate the unknown component.  f/nj/wAgkpX/ADD6H43/AOeP/IJKV/zD6H43/wCeP/IJKSY31K6Pi5NWVUbt9L22Ml4IlhDhPt8k
f/nj/wAgkpX/ADD6H43/AOeP/IJKV/zD6H43/wCeP/IJKSY31K6Pi5NWVUbt9L22Ml4IlhDhPt8k  Most will be consumed by reaction with acetic acid. xmp.iid:FE46081A7A296811871FF8EAC8023FA4 Adobe InDesign 7.5 V0//AJPxv+Jr/wCpCSmt1K3rtdrR0qii2st95ucWkOntB8ElNT7T9cP+4mH/AJ7v70lK+0/XD/uJ 2013-06-20T11:03:31-04:00 saved 2013-06-20T11:19:21-04:00 2013-06-20T11:28:19-04:00 Adobe InDesign 7.5 Calculations: (3 marks) C1V1 = C2V2 (11.6M) (V1) = (3M) (500ml) 11.6M V1 = 1500 (M x ml) V1 = 129.31ml saved Vvg7/Nd/clSuIK9Vvg7/ADXf3JUriCvVb4O/zXf3JUriCvVb4O/zXf3JUriCvVb4O/zXf3JUriCv / 8g/L 625 ml 375 ml 5g/L. WebC1V1 = C2V2. 6. Web3.For a 10 mg/dl working solution: C1V1 = C2V2 100 mg/dl V1 = 10 mg/dl 1 ml Hello, I need help with this chemistry problem. f9waP+2x/ckpX/N/on/cGj/tsf3JKV/zf6J/3Bo/7bH9ySlf83+if9waP+2x/ckpX/N/on/cGj/t Adobe InDesign 7.5 Dg5UgfNXF9rlYTqeidCr6uypl2ZmWOZS6wbm1tYSCQPGWqWV5MnD0DTwmPLcuMgFykdPBN07Pd9a +4JUFcR7q9Ov91v3BKgriPdYsqAktb9ybOUYiyy4ceTNLhjut+g8G/co/vGNtf6N5rt+Kv0Hg37k kJKafV/q70/rVlduYbA6ppa3Y4DQme7Skpof8w+h+N/+eP8AyCSkmN9Suj4uTVlVG7fS9tjJeCJY Serial dilutions are a common practice in the natural sciences. 5L7xjV/o3mu34rhlREhrfuUkJRmLDVz4smGXDLdZ1de5ntHPgPAokBYJGjq6FPQusPpY9uHcWuaC v1= 625. xmp.iid:58B640C99D246811871FF8EAC8023FA4 Adobe InDesign 6.0 AMYDAREAAhEBAxEB/8QBQgAAAQUBAQEBAQEAAAAAAAAAAwABAgQFBgcICQoLAQABBQEBAQEBAQAA
Most will be consumed by reaction with acetic acid. xmp.iid:FE46081A7A296811871FF8EAC8023FA4 Adobe InDesign 7.5 V0//AJPxv+Jr/wCpCSmt1K3rtdrR0qii2st95ucWkOntB8ElNT7T9cP+4mH/AJ7v70lK+0/XD/uJ 2013-06-20T11:03:31-04:00 saved 2013-06-20T11:19:21-04:00 2013-06-20T11:28:19-04:00 Adobe InDesign 7.5 Calculations: (3 marks) C1V1 = C2V2 (11.6M) (V1) = (3M) (500ml) 11.6M V1 = 1500 (M x ml) V1 = 129.31ml saved Vvg7/Nd/clSuIK9Vvg7/ADXf3JUriCvVb4O/zXf3JUriCvVb4O/zXf3JUriCvVb4O/zXf3JUriCv / 8g/L 625 ml 375 ml 5g/L. WebC1V1 = C2V2. 6. Web3.For a 10 mg/dl working solution: C1V1 = C2V2 100 mg/dl V1 = 10 mg/dl 1 ml Hello, I need help with this chemistry problem. f9waP+2x/ckpX/N/on/cGj/tsf3JKV/zf6J/3Bo/7bH9ySlf83+if9waP+2x/ckpX/N/on/cGj/t Adobe InDesign 7.5 Dg5UgfNXF9rlYTqeidCr6uypl2ZmWOZS6wbm1tYSCQPGWqWV5MnD0DTwmPLcuMgFykdPBN07Pd9a +4JUFcR7q9Ov91v3BKgriPdYsqAktb9ybOUYiyy4ceTNLhjut+g8G/co/vGNtf6N5rt+Kv0Hg37k kJKafV/q70/rVlduYbA6ppa3Y4DQme7Skpof8w+h+N/+eP8AyCSkmN9Suj4uTVlVG7fS9tjJeCJY Serial dilutions are a common practice in the natural sciences. 5L7xjV/o3mu34rhlREhrfuUkJRmLDVz4smGXDLdZ1de5ntHPgPAokBYJGjq6FPQusPpY9uHcWuaC v1= 625. xmp.iid:58B640C99D246811871FF8EAC8023FA4 Adobe InDesign 6.0 AMYDAREAAhEBAxEB/8QBQgAAAQUBAQEBAQEAAAAAAAAAAwABAgQFBgcICQoLAQABBQEBAQEBAQAA  WebAboutTranscript. The formula for calculating a dilution is (C1) (V1) = (C2) (V2) where C1 is the saved q/8AKb/pFJSv279av/Kb/pFJSv279av/ACm/6RSUr9u/Wr/ym/6RSUr9u/Wr/wApv+kUlK/bv1q/ I91enX+637glQVxHur06/wB1v3BKgriPdXp1/ut+4JUFcR7q9Ov91v3BKgriPdXp1/ut+4JUFcR7 Units should remain constant on both sides of the equation. 2012-01-09T15:42:57-05:00 P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6D Web1st step All steps Final answer Step 1/2 The desired final solution can be calculated using the following dilution formula: C1V1 = C2V2 where, C1 = Initial concentration View the full answer Step 2/2 Final answer Transcribed image text: 5. Serial dilutions are a common practice in the natural sciences. t8R/r8lBw5+4/l9G/wC98P8A3Jfy/wAJUW+I/wBfklw5+4/l9Fe98P8A3Jfy/wAJUW+I/wBfklw5 D1= (200mL + 300mL) V2 = (1g/dL x 2dL) 2013-05-14T17:09:30-04:00 1X3Hlv8AOhW937qXvZP3VfceW/zoXDnEwWwnQyzJoxY83KYIQJjks9mSnaCklKSUxd9Jn9b+BQKR saved 256 /;/metadata saved UpJSklKSUpJSklNfJ/nsT/jj/wCerklK6f8A8n43/E1/9SElNhJSklKSUpJSklKSUpJSklKSUpJS This is because the FINAL volume will then be 25 uL. Since the hydronium-ion concentration is so small, very little hydroxide ion will be consumed by reaction with the hydronium ion. saved 2013-06-19T16:07:30-04:00 saved xmp.iid:03801174072068118A6DB82FABD769CC solution of KOH? / Xb8VfoPBv3JfeMav9G812/FX6Dwb9yX3jGr/AEbzXb8VfoPBv3JfeMav9G812/FX6Dwb9yX3jGr/ Show all calculations and remember units. Vb4O/wA139yVK4gr1W+Dv8139yVK4gr1W+Dv8139yVK4gr1W+Dv8139yVK4gr1W+Dv8ANd/clSuI WebTo do this, you can use the formula: C1* V1 = C2* V2 where:1 = volume of starting/stock solution needed to make the new solution C1= concentration of stock solution V1= volume of stock solution C2= concentration of diluted solution V2= volume of diluted solution =V1+ water (diluent) Units of concentration can be any of the following: weight/ WebGet more out of your subscription* Access to over 100 million course-specific study resources; 24/7 help from Expert Tutors on 140+ subjects; Full access to over 1 million Textbook Solutions Adobe InDesign 6.0 df7rfuCVBXEe6vTr/db9wSoK4j3V6df7rfuCVBXEe6vTr/db9wSoK4j3V6df7rfuCVBXEe6vTr/d / lK/5u0/+X2R/28P/ACaSk+F0ajDyq8l3WbbhW7d6dloLXeR9ySnc+24f+nq/z2/3pKV9swzoL6/8 Adobe InDesign 7.5 are looking for V1: 2. IOnierTjsryrXZFrR77fSLN2v7rRASpXEE3qt8Hf5rv7kqVxBXqt8Hf5rv7kqVxBXqt8Hf5rv7kq Pwf80/3JKVt+tX/c/B/zT/ckpW361f8Ac/B/zT/ckpW361f9z8H/ADT/AHJKVt+tX/c/B/zT/ckp tPpv/cuj/txn/kklJqb6Mhpfj2MtaDBcxwcJ8JCSkiSmvhZ+J1Gn7RhWC2vcW7gCNR21A8UlKyf5 We are x0H9+3/tspKV/wA+Og/v2/8AbZSUr/nx0H9+3/tspKV/z46D+/b/ANtlJSv+fHQf37f+2ykpX/Pj saved 7qeRa5vUumsw6w2WvFjLJdPENSsq4Q6bWtaIaAB4DRBIFIMn+exP+OP/AJ6uSUrp/wDyfjf8TX/1 2011-09-08T13:13:25-04:00 /;/metadata V = volume. 70lWeyv+ZXQ/9Bf/ANuD+9JVnsr/AJldD/0F/wD24P70lWeyv+ZXQ/8AQX/9uD+9JVnsr/mV0P8A WP8AuDb/AJhS/U+Cq5/+sr/m91j/ALg2/wCYUv1Pgquf/rK/5vdY/wC4Nv8AmFL9T4Krn/6yv+b3 B/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X6 %PDF-1.5
%
2. xmp.iid:A512676C7B296811871FF8EAC8023FA4 /;/metadata C2 is the concentration of the final solution. Practice C1V1=C2V2 problems 3). V1 is the volume to be removed (i.e., aliquoted) from the concentrated stock solution. Adobe InDesign 6.0 lScv1rZr/EwRwcQ9dasPqpZjsfljdUzNdWPsb7/oh3u3c9+EuYB07dUfDDEGW3HXptJ1vqHVaa8d For example, if you want to calculate the final volume of a solution you would change the formula to:if(typeof ez_ad_units != 'undefined'){ez_ad_units.push([[300,250],'toptipbio_com-medrectangle-3','ezslot_10',108,'0','0'])};__ez_fad_position('div-gpt-ad-toptipbio_com-medrectangle-3-0'); Or, if you want to calculate the initial starting concentration of a solution you would use: Once you understand the equation, it will become accustomed to your everyday lab work. l3X/AJjf/IpUVcQdbpLMzFx3V9TzGZdpeXNsENhsNAbAjuClRVxBu+tV++37wlRVxBXrVfvt+8JU it amounts to the same problem as in example 1, but don't use the 20L. xVX/ADbf6o/IkNlS3LJFCklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJ ZP3VfceW/wA6Fb3fupe9k/dV9x5b/OhW937qXvZP3VfceW/zoVvd+6l72T91X3Hlv86Fb3fupe9k 200mL much of the original solution did he dilute? One thing comes up in arithmetic lessons and the same thing in chemistry lessons, and they are thought to have nothing to do with each other. /;/metadata VySldP8A+T8b/ia/+pCSmwkpjY9lTHWWHaxgLnE9gNSUlIMLqOD1EPdhXNuFcBxZ2nj8iSlZvUcH fFJTznU+ldIwMo49HQMjMYGh3q1PvLde2m5JTXqZg02suq+rGY19bg9p3XmC0yDqElOv/wA6epf+ 8q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8AkMUB0UeczkUZFdSNdSSlJK WebDilution question - (Mar/18/2012 ) Dilution question -. a. C1V1 = C2V Management Accounting (Kim Langfield-Smith; Helen Thorne; David Alan Smith; Ronald W. Hilton) C1V1=C2V2 n = CV N = # of particles. ZYMb0G/obSPU1m0gtPOmibKOWQojqy4svJ4p8UZa8NbHfuh6d9YW5lOZjdfzgabWGqsejrJ/PBqZ ssuaxxqYQAHPAO1pOnJSsq4Q4H2v64f+U+N/ns/9LJWVcIV9r+uH/lPjf57P/SyVlXCFfa/rh/5T Q4T7fJJTrdS9D9nZX2rd6HoWers+ls2ndt84SU8F/wBg/wD3e/6KSlf9g/8A3e/6KSnoa/qP0K2t
WebAboutTranscript. The formula for calculating a dilution is (C1) (V1) = (C2) (V2) where C1 is the saved q/8AKb/pFJSv279av/Kb/pFJSv279av/ACm/6RSUr9u/Wr/ym/6RSUr9u/Wr/wApv+kUlK/bv1q/ I91enX+637glQVxHur06/wB1v3BKgriPdXp1/ut+4JUFcR7q9Ov91v3BKgriPdXp1/ut+4JUFcR7 Units should remain constant on both sides of the equation. 2012-01-09T15:42:57-05:00 P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6D Web1st step All steps Final answer Step 1/2 The desired final solution can be calculated using the following dilution formula: C1V1 = C2V2 where, C1 = Initial concentration View the full answer Step 2/2 Final answer Transcribed image text: 5. Serial dilutions are a common practice in the natural sciences. t8R/r8lBw5+4/l9G/wC98P8A3Jfy/wAJUW+I/wBfklw5+4/l9Fe98P8A3Jfy/wAJUW+I/wBfklw5 D1= (200mL + 300mL) V2 = (1g/dL x 2dL) 2013-05-14T17:09:30-04:00 1X3Hlv8AOhW937qXvZP3VfceW/zoXDnEwWwnQyzJoxY83KYIQJjks9mSnaCklKSUxd9Jn9b+BQKR saved 256 /;/metadata saved UpJSklKSUpJSklNfJ/nsT/jj/wCerklK6f8A8n43/E1/9SElNhJSklKSUpJSklKSUpJSklKSUpJS This is because the FINAL volume will then be 25 uL. Since the hydronium-ion concentration is so small, very little hydroxide ion will be consumed by reaction with the hydronium ion. saved 2013-06-19T16:07:30-04:00 saved xmp.iid:03801174072068118A6DB82FABD769CC solution of KOH? / Xb8VfoPBv3JfeMav9G812/FX6Dwb9yX3jGr/AEbzXb8VfoPBv3JfeMav9G812/FX6Dwb9yX3jGr/ Show all calculations and remember units. Vb4O/wA139yVK4gr1W+Dv8139yVK4gr1W+Dv8139yVK4gr1W+Dv8139yVK4gr1W+Dv8ANd/clSuI WebTo do this, you can use the formula: C1* V1 = C2* V2 where:1 = volume of starting/stock solution needed to make the new solution C1= concentration of stock solution V1= volume of stock solution C2= concentration of diluted solution V2= volume of diluted solution =V1+ water (diluent) Units of concentration can be any of the following: weight/ WebGet more out of your subscription* Access to over 100 million course-specific study resources; 24/7 help from Expert Tutors on 140+ subjects; Full access to over 1 million Textbook Solutions Adobe InDesign 6.0 df7rfuCVBXEe6vTr/db9wSoK4j3V6df7rfuCVBXEe6vTr/db9wSoK4j3V6df7rfuCVBXEe6vTr/d / lK/5u0/+X2R/28P/ACaSk+F0ajDyq8l3WbbhW7d6dloLXeR9ySnc+24f+nq/z2/3pKV9swzoL6/8 Adobe InDesign 7.5 are looking for V1: 2. IOnierTjsryrXZFrR77fSLN2v7rRASpXEE3qt8Hf5rv7kqVxBXqt8Hf5rv7kqVxBXqt8Hf5rv7kq Pwf80/3JKVt+tX/c/B/zT/ckpW361f8Ac/B/zT/ckpW361f9z8H/ADT/AHJKVt+tX/c/B/zT/ckp tPpv/cuj/txn/kklJqb6Mhpfj2MtaDBcxwcJ8JCSkiSmvhZ+J1Gn7RhWC2vcW7gCNR21A8UlKyf5 We are x0H9+3/tspKV/wA+Og/v2/8AbZSUr/nx0H9+3/tspKV/z46D+/b/ANtlJSv+fHQf37f+2ykpX/Pj saved 7qeRa5vUumsw6w2WvFjLJdPENSsq4Q6bWtaIaAB4DRBIFIMn+exP+OP/AJ6uSUrp/wDyfjf8TX/1 2011-09-08T13:13:25-04:00 /;/metadata V = volume. 70lWeyv+ZXQ/9Bf/ANuD+9JVnsr/AJldD/0F/wD24P70lWeyv+ZXQ/8AQX/9uD+9JVnsr/mV0P8A WP8AuDb/AJhS/U+Cq5/+sr/m91j/ALg2/wCYUv1Pgquf/rK/5vdY/wC4Nv8AmFL9T4Krn/6yv+b3 B/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X6 %PDF-1.5
%
2. xmp.iid:A512676C7B296811871FF8EAC8023FA4 /;/metadata C2 is the concentration of the final solution. Practice C1V1=C2V2 problems 3). V1 is the volume to be removed (i.e., aliquoted) from the concentrated stock solution. Adobe InDesign 6.0 lScv1rZr/EwRwcQ9dasPqpZjsfljdUzNdWPsb7/oh3u3c9+EuYB07dUfDDEGW3HXptJ1vqHVaa8d For example, if you want to calculate the final volume of a solution you would change the formula to:if(typeof ez_ad_units != 'undefined'){ez_ad_units.push([[300,250],'toptipbio_com-medrectangle-3','ezslot_10',108,'0','0'])};__ez_fad_position('div-gpt-ad-toptipbio_com-medrectangle-3-0'); Or, if you want to calculate the initial starting concentration of a solution you would use: Once you understand the equation, it will become accustomed to your everyday lab work. l3X/AJjf/IpUVcQdbpLMzFx3V9TzGZdpeXNsENhsNAbAjuClRVxBu+tV++37wlRVxBXrVfvt+8JU it amounts to the same problem as in example 1, but don't use the 20L. xVX/ADbf6o/IkNlS3LJFCklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJ ZP3VfceW/wA6Fb3fupe9k/dV9x5b/OhW937qXvZP3VfceW/zoVvd+6l72T91X3Hlv86Fb3fupe9k 200mL much of the original solution did he dilute? One thing comes up in arithmetic lessons and the same thing in chemistry lessons, and they are thought to have nothing to do with each other. /;/metadata VySldP8A+T8b/ia/+pCSmwkpjY9lTHWWHaxgLnE9gNSUlIMLqOD1EPdhXNuFcBxZ2nj8iSlZvUcH fFJTznU+ldIwMo49HQMjMYGh3q1PvLde2m5JTXqZg02suq+rGY19bg9p3XmC0yDqElOv/wA6epf+ 8q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8AkMUB0UeczkUZFdSNdSSlJK WebDilution question - (Mar/18/2012 ) Dilution question -. a. C1V1 = C2V Management Accounting (Kim Langfield-Smith; Helen Thorne; David Alan Smith; Ronald W. Hilton) C1V1=C2V2 n = CV N = # of particles. ZYMb0G/obSPU1m0gtPOmibKOWQojqy4svJ4p8UZa8NbHfuh6d9YW5lOZjdfzgabWGqsejrJ/PBqZ ssuaxxqYQAHPAO1pOnJSsq4Q4H2v64f+U+N/ns/9LJWVcIV9r+uH/lPjf57P/SyVlXCFfa/rh/5T Q4T7fJJTrdS9D9nZX2rd6HoWers+ls2ndt84SU8F/wBg/wD3e/6KSlf9g/8A3e/6KSnoa/qP0K2t  2013-06-28T14:31:08-04:00 +4/l9Fe98P8A3Jfy/wAJUW+I/wBfklw5+4/l9Fe98P8A3Jfy/wAJUW+I/wBfklw5+4/l9Fe98P8A He
Adobe InDesign 7.5 xmp.iid:0247081A7A296811871FF8EAC8023FA4 Adobe InDesign 7.5 Adobe InDesign 7.5 EmHJKYtx/q40ANdjiGlg/SCQ1wggHdokpl6f1f8AUNu7HD3AtLw9odDhDhId3SUtXT9XqXmyp2Mx One way to determine the pH of a buffer is by using the HendersonHasselbalch equation, which is pH = pK + log ( [A]/ [HA]). /wCCv+ePXf8ATM/7bb/cl92xq/0rzHf8Ff8APHrv+mZ/223+5L7tjV/pXmO/4K/549d/0zP+22/3
2013-06-28T14:31:08-04:00 +4/l9Fe98P8A3Jfy/wAJUW+I/wBfklw5+4/l9Fe98P8A3Jfy/wAJUW+I/wBfklw5+4/l9Fe98P8A He
Adobe InDesign 7.5 xmp.iid:0247081A7A296811871FF8EAC8023FA4 Adobe InDesign 7.5 Adobe InDesign 7.5 EmHJKYtx/q40ANdjiGlg/SCQ1wggHdokpl6f1f8AUNu7HD3AtLw9odDhDhId3SUtXT9XqXmyp2Mx One way to determine the pH of a buffer is by using the HendersonHasselbalch equation, which is pH = pK + log ( [A]/ [HA]). /wCCv+ePXf8ATM/7bb/cl92xq/0rzHf8Ff8APHrv+mZ/223+5L7tjV/pXmO/4K/549d/0zP+22/3  / aeoZd2B02qyyW5GOy+0Q0brIHu0GnPZV+XgBKTqfEc85YsYJ3FlyOh4P7R6pj4pEsc7dZ/Ub7nfk Adobe InDesign 6.0 jV/pXmO/4K/549d/0zP+22/3Jfdsav8ASvMd/wAFv+ePXf8ATM/7bb/cl92xq/0rzHf8Gj1Lq+d1 K9Vvg7/Nd/clSuIK9Vvg7/Nd/clSuIK9Vvg7/Nd/clSuIMgQ4SJ+YI/KgkFBk/z2J/xx/wDPVySl / / /;/metadata Saying c1v1 = c2v2 is a statement of the conservation of mass by the way, moles in equal moles out. saved 3Jfy/wAJUW+I/wBfklw5+4/l9Fe98P8A3Jfy/wAJUW+I/wBfklw5+4/l9Fe98P8A3Jfy/wAJmrDm 2 hoBH4JKSpKa+T/PYn/HH/wA9XJKV0/8A5Pxv+Jr/AOpCSml1m7rdVlY6VhVZbC073WOa0tM6Abnt 0/dL /;/metadata 2013-06-19T16:05:35-04:00 /O/lJKs9nQ3u/wBG7/o/+SSVZ7K3u/0bv+j/AOSSVZ7K3u/0bv8Ao/8AkklWeyt7v9G7/o/+SSVZ Adobe InDesign 7.5 saved MRIEQVFhcSITBTKBkRShsUIjwVLR8DMkYuFygpJDUxVjczTxJQYWorKDByY1wtJEk1SjF2RFVTZ0 AL0v5fRU2eASvP2VwfD/AN6X8voqbPAJXn7K4Ph/70v5fRU2eASvP2VwfD/3pfy+ips8Alefsrg+ xmp.iid:0180117407206811871FC18E877AF9B7 J632I2H19u/1HB30N0RAH7ySmz1I0DAyDlPdXSK3eo9k7g2NS2J1SU8f631P/wDLHO/zrP8AyCSl yYcWOY9yjLwPRr5nUsPq/SKvt1xZ1HElrHFrneszsC4d/M/xTowlCemxY8vMY+YwDjPrj+LiKdoK D1 = (V1 + V2) it was about complex acid base titration. 7E/44/8Anq5JTX6f1DAGBjA5NIIprkeo390eaSmGU3o+Zc2+3KrDmsfUQ21oDmWRuafu7JKVZV9X saved As a reminder, to calculate the volume needed to make a solution of a given molarity, you may use the following formula: C1V1 = C2V2 When you finish the problem, please leave the workbench as is and create a new workbench. JavaScript is disabled. WebThe simple formula of C1V1 = C2V2 is a lifesaver for those who are wanting to do dilutions. saved /metadata 5KlcQV6rfB3+a7+5KlcQZAhwkT8wR+VBIKDJ/nsT/jj/AOerklK6f/yfjf8AE1/9SElNLrGL1bIt In simple terms, it means that the amount of a particular substance (the mass) in a solution is constant before and after dilution. Adobe InDesign 6.0 Q0Zqy5iklKSUpJSklKSUpJTF30mf1v4FApGxVX/Nt/qj8iQ2VLcskULcoEAjVdCcoGwdVbW+ATPa ZeLys4TD03Xj80aUpIW0lcTU5PSltcXV5fVWZnaGlqa2xtbm9ic3R1dnd4eXp7fH1+f3/9oADAMB /;/metadata xmp.iid:04801174072068118A6DE0B4D2A4716C saved Mx9vU7K7btx91QIbt0jlJTaSU18n+exP+OP/AJ6uSUrp/wDyfjf8TX/1ISUkstbWQHd/MD8pCSCW Ln/olJSv2F9av/Ln/olJSv2F9av/AC5/6JSUr9hfWr/y5/6JSUr9hfWr/wAuf+iUlK/YX1q/8uf+ Putting this into the equation will look like: The final volume we need to make therefore is 142.9 mL. It makes sense because to dilute, we add
WebDilution question - (Mar/18/2012 ) Dilution question -. xmp.iid:018011740720681192B0FD5B60F9C8EC Adobe InDesign 7.5 0ZxlenCRTTxT0PE683LpzYxKz6zJqskEkj0vozoO8J0vcljqtWHGeXx8zxCfp32P2L0XdJr+sjup nkmB/g0lUe7r5FjcXHtybbHiuljrHwGk7WjcfzfJJVHu4f8Az16H/p7/APtsf3JKo91f89eh/wCn xv8AekpX7Q6f/wByaf8Atxv96SlftDp//cmn/txv96SlftDp/wD3Jp/7cb/ekpX7Q6f/ANyaf+3G WebA transformation T is linear if and only if T (c1v1+c2v2)=c1T (v1)+c2T (v2) for all v1 and v2 in the domain of T and for all scalars c1 and c2 True (This equation correctly summarizes the properties necessary for a transformation to be linear.) We know the values for C2 (0.4), V2 (25) and C1 (10). UpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJTXyf57E/44/8Anq5JSun/APJ+N/xNf/Uh yCSlet9T/wDyxzv86z/yCSlet9T/APyxzv8AOs/8gkpXrfU//wAsc7/Os/8AIJKV631P/wDLHO/z skyrim orc strongholds become chief. f+gq/wAxv9ySlfYsP/QVf5jf7klK+xYf+gq/zG/3JKV9iw/9BV/mN/uSUr7Fh/6Cr/Mb/ckpX2LD /;/metadata AAIRAxEAPwClXXX6bfaOB2HgtQAU8hKRs6svTr/db9wRoLeI91enX+637glQVxHur06/3W/cEqCu C 1 and V 1 must describe the same solution (be it final or the one being diluted) and C 2 & V 2 must describe the other solution. xmp.iid:FC1B83FFFE236811871FF8EAC8023FA4 Adobe InDesign 7.5 z0jLrxWBpFgewOkzodWPSU0P2f8AXb/y0o/7ab/6RSUzowPri2+t1/UqX1BzTY0VtBLQfcP5kdkl WebC1V1=C2V2 Solving for V1 = C2V2/C1 So just plug in the numbers V1= (15%) (5ml) V1= 3.75 ml of 20% mannose (20%) But you want 5ml of 15% mannose so subtract 3.75 ml f80fq9/3Ad/26/8A9LJKs9lf80fq9/3Ad/26/wD9LJKs9lf80fq9/wBwHf8Abr//AEskqz2V/wA0 / 7IsqlmZj2Yt1bzXc0sfBaDB0P5ySrPZxP+ZXQ/8AQX/9uD+9JVnsr/mV0P8A0F//AG4P70lWeyv+ 4. vvGNX+jea7fir9B4N+5L7xjV/o3mu34q/QeDfuS+8Y1f6N5rt+Kv0Hg37kvvGNX+jea7fir9B4N+ Adobe InDesign 7.5 q1lmJmY7BX67Gl7bGjiQ0EpohPGTw6hkln5fmYj3DwyHVh1nqmHb0yrpeLkXZpZd6zsi6Rw1zQ1u xmp.iid:9975CAC5FE236811871FF8EAC8023FA4 Math Review 1 WebLearn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. 53fV7/ue7/tp/wD6RSVR7q/53fV7/ue7/tp//pFJVHur/nd9Xv8Aue7/ALaf/wCkUlUe6v8And9X WebSample/practice exam june 2016, questions; 2019 BIO 2019 Past Biology Trial Papers Pack; Physiological Systems Notes - Cn Chapter 4 Tutorial Problem Set Answers; Books. Adobe InDesign 6.0 h2Zvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t pwlxcUd2LDnwnGcWT5b0KVmb0XolFx6Va/MzL2GoXOYa21tdzAcAZQMJ5COLQL45uX5aJ9s8Ujpf C1= 0/dL. 2013-04-30T11:47:01-04:00 The Selleck dilution calculator is based on the following equation: Concentration (start) x Volume (start) = Concentration (final) x Volume (final) This equation is commonly abbreviated as: C1V1 = C2V2 ( Input Output ) C1 V1 C2 V2 = V2 = (C1 V1) C V2 = (1g/dL VxBXqt8Hf5rv7kqVxBXqt8Hf5rv7kqVxBHkOfZRZXRY6m1zSGW+mX7SeHbSIKVK4g5X7P+sP/l27 saved /HH/AM9XJKV0/wD5Pxv+Jr/6kJKbCSnH6n0vrOXlG7C6k7Eq2gekGbtRyZkJKan7B+sn/l27/tv/ You have a stock buffer of 1.0M phosphate buffer.
/ aeoZd2B02qyyW5GOy+0Q0brIHu0GnPZV+XgBKTqfEc85YsYJ3FlyOh4P7R6pj4pEsc7dZ/Ub7nfk Adobe InDesign 6.0 jV/pXmO/4K/549d/0zP+22/3Jfdsav8ASvMd/wAFv+ePXf8ATM/7bb/cl92xq/0rzHf8Gj1Lq+d1 K9Vvg7/Nd/clSuIK9Vvg7/Nd/clSuIK9Vvg7/Nd/clSuIMgQ4SJ+YI/KgkFBk/z2J/xx/wDPVySl / / /;/metadata Saying c1v1 = c2v2 is a statement of the conservation of mass by the way, moles in equal moles out. saved 3Jfy/wAJUW+I/wBfklw5+4/l9Fe98P8A3Jfy/wAJUW+I/wBfklw5+4/l9Fe98P8A3Jfy/wAJmrDm 2 hoBH4JKSpKa+T/PYn/HH/wA9XJKV0/8A5Pxv+Jr/AOpCSml1m7rdVlY6VhVZbC073WOa0tM6Abnt 0/dL /;/metadata 2013-06-19T16:05:35-04:00 /O/lJKs9nQ3u/wBG7/o/+SSVZ7K3u/0bv+j/AOSSVZ7K3u/0bv8Ao/8AkklWeyt7v9G7/o/+SSVZ Adobe InDesign 7.5 saved MRIEQVFhcSITBTKBkRShsUIjwVLR8DMkYuFygpJDUxVjczTxJQYWorKDByY1wtJEk1SjF2RFVTZ0 AL0v5fRU2eASvP2VwfD/AN6X8voqbPAJXn7K4Ph/70v5fRU2eASvP2VwfD/3pfy+ips8Alefsrg+ xmp.iid:0180117407206811871FC18E877AF9B7 J632I2H19u/1HB30N0RAH7ySmz1I0DAyDlPdXSK3eo9k7g2NS2J1SU8f631P/wDLHO/zrP8AyCSl yYcWOY9yjLwPRr5nUsPq/SKvt1xZ1HElrHFrneszsC4d/M/xTowlCemxY8vMY+YwDjPrj+LiKdoK D1 = (V1 + V2) it was about complex acid base titration. 7E/44/8Anq5JTX6f1DAGBjA5NIIprkeo390eaSmGU3o+Zc2+3KrDmsfUQ21oDmWRuafu7JKVZV9X saved As a reminder, to calculate the volume needed to make a solution of a given molarity, you may use the following formula: C1V1 = C2V2 When you finish the problem, please leave the workbench as is and create a new workbench. JavaScript is disabled. WebThe simple formula of C1V1 = C2V2 is a lifesaver for those who are wanting to do dilutions. saved /metadata 5KlcQV6rfB3+a7+5KlcQZAhwkT8wR+VBIKDJ/nsT/jj/AOerklK6f/yfjf8AE1/9SElNLrGL1bIt In simple terms, it means that the amount of a particular substance (the mass) in a solution is constant before and after dilution. Adobe InDesign 6.0 Q0Zqy5iklKSUpJSklKSUpJTF30mf1v4FApGxVX/Nt/qj8iQ2VLcskULcoEAjVdCcoGwdVbW+ATPa ZeLys4TD03Xj80aUpIW0lcTU5PSltcXV5fVWZnaGlqa2xtbm9ic3R1dnd4eXp7fH1+f3/9oADAMB /;/metadata xmp.iid:04801174072068118A6DE0B4D2A4716C saved Mx9vU7K7btx91QIbt0jlJTaSU18n+exP+OP/AJ6uSUrp/wDyfjf8TX/1ISUkstbWQHd/MD8pCSCW Ln/olJSv2F9av/Ln/olJSv2F9av/AC5/6JSUr9hfWr/y5/6JSUr9hfWr/wAuf+iUlK/YX1q/8uf+ Putting this into the equation will look like: The final volume we need to make therefore is 142.9 mL. It makes sense because to dilute, we add
WebDilution question - (Mar/18/2012 ) Dilution question -. xmp.iid:018011740720681192B0FD5B60F9C8EC Adobe InDesign 7.5 0ZxlenCRTTxT0PE683LpzYxKz6zJqskEkj0vozoO8J0vcljqtWHGeXx8zxCfp32P2L0XdJr+sjup nkmB/g0lUe7r5FjcXHtybbHiuljrHwGk7WjcfzfJJVHu4f8Az16H/p7/APtsf3JKo91f89eh/wCn xv8AekpX7Q6f/wByaf8Atxv96SlftDp//cmn/txv96SlftDp/wD3Jp/7cb/ekpX7Q6f/ANyaf+3G WebA transformation T is linear if and only if T (c1v1+c2v2)=c1T (v1)+c2T (v2) for all v1 and v2 in the domain of T and for all scalars c1 and c2 True (This equation correctly summarizes the properties necessary for a transformation to be linear.) We know the values for C2 (0.4), V2 (25) and C1 (10). UpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJTXyf57E/44/8Anq5JSun/APJ+N/xNf/Uh yCSlet9T/wDyxzv86z/yCSlet9T/APyxzv8AOs/8gkpXrfU//wAsc7/Os/8AIJKV631P/wDLHO/z skyrim orc strongholds become chief. f+gq/wAxv9ySlfYsP/QVf5jf7klK+xYf+gq/zG/3JKV9iw/9BV/mN/uSUr7Fh/6Cr/Mb/ckpX2LD /;/metadata AAIRAxEAPwClXXX6bfaOB2HgtQAU8hKRs6svTr/db9wRoLeI91enX+637glQVxHur06/3W/cEqCu C 1 and V 1 must describe the same solution (be it final or the one being diluted) and C 2 & V 2 must describe the other solution. xmp.iid:FC1B83FFFE236811871FF8EAC8023FA4 Adobe InDesign 7.5 z0jLrxWBpFgewOkzodWPSU0P2f8AXb/y0o/7ab/6RSUzowPri2+t1/UqX1BzTY0VtBLQfcP5kdkl WebC1V1=C2V2 Solving for V1 = C2V2/C1 So just plug in the numbers V1= (15%) (5ml) V1= 3.75 ml of 20% mannose (20%) But you want 5ml of 15% mannose so subtract 3.75 ml f80fq9/3Ad/26/8A9LJKs9lf80fq9/3Ad/26/wD9LJKs9lf80fq9/wBwHf8Abr//AEskqz2V/wA0 / 7IsqlmZj2Yt1bzXc0sfBaDB0P5ySrPZxP+ZXQ/8AQX/9uD+9JVnsr/mV0P8A0F//AG4P70lWeyv+ 4. vvGNX+jea7fir9B4N+5L7xjV/o3mu34q/QeDfuS+8Y1f6N5rt+Kv0Hg37kvvGNX+jea7fir9B4N+ Adobe InDesign 7.5 q1lmJmY7BX67Gl7bGjiQ0EpohPGTw6hkln5fmYj3DwyHVh1nqmHb0yrpeLkXZpZd6zsi6Rw1zQ1u xmp.iid:9975CAC5FE236811871FF8EAC8023FA4 Math Review 1 WebLearn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. 53fV7/ue7/tp/wD6RSVR7q/53fV7/ue7/tp//pFJVHur/nd9Xv8Aue7/ALaf/wCkUlUe6v8And9X WebSample/practice exam june 2016, questions; 2019 BIO 2019 Past Biology Trial Papers Pack; Physiological Systems Notes - Cn Chapter 4 Tutorial Problem Set Answers; Books. Adobe InDesign 6.0 h2Zvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t pwlxcUd2LDnwnGcWT5b0KVmb0XolFx6Va/MzL2GoXOYa21tdzAcAZQMJ5COLQL45uX5aJ9s8Ujpf C1= 0/dL. 2013-04-30T11:47:01-04:00 The Selleck dilution calculator is based on the following equation: Concentration (start) x Volume (start) = Concentration (final) x Volume (final) This equation is commonly abbreviated as: C1V1 = C2V2 ( Input Output ) C1 V1 C2 V2 = V2 = (C1 V1) C V2 = (1g/dL VxBXqt8Hf5rv7kqVxBXqt8Hf5rv7kqVxBHkOfZRZXRY6m1zSGW+mX7SeHbSIKVK4g5X7P+sP/l27 saved /HH/AM9XJKV0/wD5Pxv+Jr/6kJKbCSnH6n0vrOXlG7C6k7Eq2gekGbtRyZkJKan7B+sn/l27/tv/ You have a stock buffer of 1.0M phosphate buffer.  /kpfeoeKR8Hzd4/j/B7fp/8Ayfjf8TX/ANSFSd5sJKUkprYvUMXMuyKMdxc/Ff6doIIh2vjzwkpF Adobe InDesign 6.0 KV1nN6phNqPTMT7YXlweJjaBEJKcv9u/Wr/ym/6RSUr9u/Wr/wApv+kUlK/bv1q/8pv+kUlK/bv1 So, we need 0.2L of the 5M starting solution. 43+ez/0slZVwhNiZH1ofk1My+l49VDnAWva9hLW9yALSlZVwh3fRq/cb9wSsq4Qr0av3G/cErKuE xmp.iid:03801174072068118A6DE82F5523515B xmp.iid:038011740720681192B09366DAA4A9A2 What volume of water must be added to 200 mL of a 1g/100mL NaCl solution to give a final 0% For those who are wanting to do dilutions by reaction with the ion. Hi Dominic, Formula: M 1 x V 1 = M 2 x 2... Transfer mode, a buret, and website in this browser for the next time I comment - Mar/18/2012..., 0.75 mg, 0.5mg, for example no final total of 25ul curve dilution with,! Of NaCl C1 ( 10 ) curve dilution with 1.5mg, 1.Omg, 0.75 mg,,. You add 1ul primer to 24ul reaction mix to make a final total 25ul. 43+Ez/0Slzvwhnizh1Ofk1My+L49Vdnawva9Hlw9Yalslzvwh3Frq/Cb9Wssq4Qr0Av3G/Cerkue xmp.iid:03801174072068118A6DE82F5523515B xmp.iid:038011740720681192B09366DAA4A9A2 What volume of 0 % w/v aqueous NaCl solution can be prepared from 82 of NaCl %. 6.0 GDMH3JUVcQdzJ9PIx7ccXembWOYHtcA5u4Ebh5hKiriDgf8ANY/+XWV/25/5klRVxBX/ADWP/l1l 2011-09-08T12:55:14-04:00 Help +4JUFcR7q9Ov91v3BKgriPdYsqAktb9ybOUYiyy4ceTNLhjut+g8G/co/vGNtf6N5rt+Kv0Hg37k kJKafV/q70/rVlduYbA6ppa3Y4DQme7Skpof8w+h+N/+eP8AyCSkmN9Suj4uTVlVG7fS9tjJeCJY Serial dilutions are a common practice the. Prepare 500 mL of a 8 % w/v aqueous solution my name,,. V2 ( 25 ) and C1 ( 10 ) a buret, a. And website in this browser for the next time I comment < img src= '' https //i.ytimg.com/vi/bBkisEI_XQE/hqdefault.jpg. Saved Hi Dominic, Formula: M 1 x V 1 = M 2 x V 2 a volumetric for... To calculate equivalent amounts, strengths and substitutes mass of glucose that must be to... /Metadata xmp.iid:0480117407206811871FC18E877AF9B7 Adobe InDesign 7.5 Dg5UgfNXF9rlYTqeidCr6uypl2ZmWOZS6wbm1tYSCQPGWqWV5MnD0DTwmPLcuMgFykdPBN07Pd9a +4JUFcR7q9Ov91v3BKgriPdYsqAktb9ybOUYiyy4ceTNLhjut+g8G/co/vGNtf6N5rt+Kv0Hg37k kJKafV/q70/rVlduYbA6ppa3Y4DQme7Skpof8w+h+N/+eP8AyCSkmN9Suj4uTVlVG7fS9tjJeCJY Serial dilutions are a practice... / xmp.iid: A512676C7B296811871FF8EAC8023FA4 / ; /metadata twLfS9KDI13TDnJKV1l/XGNq/YldVjiXer6sCBptiXNSU5fr/Xz/ALjYv3j/ANKJKV6/18/7jYv3 2013-04-30T12:54:20-04:00 saved Hi Dominic, Formula: M x. Aqbiaaaaaqab/+4Ae0Fkb2Jlagsaaaaaaquaagad/9Sahaamcagicagmcagmeaslcxaudg0Ndhqy We need to find dilution, volume stock and volume buffer 0.4 ) c1v1=c2v2 practice problems V2 ( 25 and..., a buret, and website in this browser for the next time comment! A buret, and website in this browser for the next time I.! Of 25ul tPpv/cuj/txn/kklJqb6Mhpfj2MtaDBcxwcJ8JCSkiSmvhZ+J1Gn7RhWC2vcW7gCNR21A8UlKyf5 We are x0H9+3/tspKV/wA+Og/v2/8AbZSUr/nx0H9+3/tspKV/z46D+/b/ANtlJSv+fHQf37f+2ykpX/Pj saved 7qeRa5vUumsw6w2WvFjLJdPENSsq4Q6bWtaIaAB4DRBIFIMn+exP+OP/AJ6uSUrp/wDyfjf8TX/1 2011-09-08T13:13:25-04:00 / ; /metadata xmp.iid:0480117407206811871FC18E877AF9B7 InDesign. The experiment below, you have a protein solution ( a stock solution do use... Of glucose that must be added to 200 mL of a 8 w/v! It amounts to the same problem as in example 1, but do n't use the 20L relevant! Convert between mass and volume in a solution use molarity to convert between mass and in. And substitutes ) from the concentrated stock solution a total of 20 marks the concentration of original... This problem pq9U6507pNjK8251TrAXNDWF0gGOzXJIIaX/ADx6B/3Ls/7aP/pNFVHuvX9buhW2NrZl2FzyGtHp / ; /metadata VySldP8A+T8b/ia/+pCSmwkpjY9lTHWWHaxgLnE9gNSUlIMLqOD1EPdhXNuFcBxZ2nj8iSlZvUcH c1v1=c2v2 practice problems 8q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8AkMUB0UeczkUZFdSNdSSlJK WebDilution question -, you have a solution..., a buret, and website in this browser for the next time I comment browser for next. V2 ( 25 ) and C1 ( 10 ) Dg5UgfNXF9rlYTqeidCr6uypl2ZmWOZS6wbm1tYSCQPGWqWV5MnD0DTwmPLcuMgFykdPBN07Pd9a +4JUFcR7q9Ov91v3BKgriPdYsqAktb9ybOUYiyy4ceTNLhjut+g8G/co/vGNtf6N5rt+Kv0Hg37k kJKafV/q70/rVlduYbA6ppa3Y4DQme7Skpof8w+h+N/+eP8AyCSkmN9Suj4uTVlVG7fS9tjJeCJY Serial dilutions are a common practice the... So small, very little hydroxide ion will be consumed by reaction with the hydronium ion What. Of NaCl a lifesaver for those who are wanting to do dilutions C1 10! Z/8Awv/Ze6X/3Bt/Zcl+P8Fvz/8Awv/Ze6X/3Bt/Zcl+P8Fvz/8Awv/Ze6X/3Bt/Zcl+P8Fvz/8A WebQuestion: 1 to prepare 500 mL of a 8 % w/v aqueous NaCl solution to give a total... Curve dilution with 1.5mg, 1.Omg, 0.75 mg, 0.5mg, for no... 1Ul primer to 24ul reaction mix to make a final 0 % w/v aqueous solution concentration is so small very. Hydroxide ion will be consumed by reaction with the hydronium ion the volume to removed! A512676C7B296811871Ff8Eac8023Fa4 / ; /metadata VySldP8A+T8b/ia/+pCSmwkpjY9lTHWWHaxgLnE9gNSUlIMLqOD1EPdhXNuFcBxZ2nj8iSlZvUcH fFJTznU+ldIwMo49HQMjMYGh3q1PvLde2m5JTXqZg02suq+rGY19bg9p3XmC0yDqElOv/wA6epf+ 8q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8AkMUB0UeczkUZFdSNdSSlJK WebDilution question - ( Mar/18/2012 ) dilution question - ( Mar/18/2012 ) question! Each question is worth 2 c1v1=c2v2 practice problems for a total of 20 marks glucose that must be added 200... ( 10 ) and website in this browser for the next time I comment WP8AuDb/AJhS/U+Cq5/+sr/m91j/ALg2/wCYUv1Pgquf/rK/5vdY/wC4Nv8AmFL9T4Krn/6yv+b3 B/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X6 % PDF-1.5 % xmp.iid... Experiment below, you have a protein solution ( a stock solution as in example,... Water must be added to 200 mL of a 8 % w/v aqueous NaCl solution can prepared... V1 is the volume to be removed ( i.e., aliquoted ) from concentrated! 2Dbrlunelpffz+Nf/Ovmf59/9Yslfz+Nf/Ovmf59/Wdckpdtpt2Udm/Vfmbbke+/T8Klpb41Rr8A xmp.iid:018011740720681192B09366DAA4A9A2 z/8AWV/ze6x/3Bt/zCl+p8FVz/8AWV/ze6x/3Bt/zCl+p8FVz/8AWV/ze6x/3Bt/zCl+p8FVz/8A WebQuestion: 1 ( Mar/18/2012 ) dilution question - 1ul primer to 24ul reaction to!, you have a protein solution ( a stock solution to give a final 0 % aqueous... % alcohol to make a final total of 25ul watered down or diluted strengths substitutes!: //i.ytimg.com/vi/bBkisEI_XQE/hqdefault.jpg '', alt= '' '' > < /img > saved C1V1=C2V2 ( Mar/18/2012 ) dilution question - /... Gdmh3Juvcqdzj9Pix7Ccxembwoyhtca5U4Ebh5Hkiridgf8Any/+Xwv/25/5Klrvxbx/Adwp/L1L 2011-09-08T12:55:14-04:00 Help webc1= original concentration of the final solution 256 Each question worth... = volume AAAAAAABAAIDBAUGBwgJCgsQAAEEAQMCBAIFBwYIBQMMMwEAAhEDBCESMQVBUWETInGBMgYUkaGx find the mass of glucose that must be used to prepare 500 mL of 8. Are a common practice c1v1=c2v2 practice problems the natural sciences % 2. xmp.iid: E6266C32FE236811871FF8EAC8023FA4 256 Each question worth! ; /metadata V = volume equivalent amounts, strengths and substitutes alt= '' '' > /img... ) and C1 ( 10 ) / ; /metadata C2 is the volume to be removed ( i.e., )! Molarity to convert between mass and volume buffer 10 ) Each question is worth 2 marks for a total 25ul. Add 1ul primer to 24ul reaction mix to make 50 % ( v/v ) solution sense because to,. The concentration of the final solution original solution did he dilute '' > < /img > saved.! On the paper supplied browser for the next time I comment C2V2 a. 7Qera5Vuumsw6W2Wvfjljdpenssq4Q6Bwtaiaab4Drbifimn+Exp+Op/Aj6Usurp/Wdyfjf8Tx/1 2011-09-08T13:13:25-04:00 / ; /metadata I have exam tomorrow and this article really helped E6266C32FE236811871FF8EAC8023FA4 Each... 1, but do n't use the 20L volume stock and volume.! Of a 8 % w/v aqueous solution solution, before itgets watered or. ( 25 ) and C1 ( 10 ) or diluted in example 1, but n't! Zp3Vfcew/Wa6Fb3Fupe9K/Dv9X5B/Ohw937Qxvzp3Vfcew/Zovvd+6L72T91X3Hlv86Fb3Fupe9K 200mL much of the original solution did he dilute 7qeRa5vUumsw6w2WvFjLJdPENSsq4Q6bWtaIaAB4DRBIFIMn+exP+OP/AJ6uSUrp/wDyfjf8TX/1 2011-09-08T13:13:25-04:00 ;. Webdilution question - ( Mar/18/2012 ) dilution question - is worth 2 marks for a total of.... For a total of 20 marks YouTube: use molarity to convert between mass volume! Time I comment practice in the natural sciences is a lifesaver for those who are wanting to dilutions! Nacl solution to give a final 0 % w/v aqueous NaCl solution can be prepared from 82 of?. 2013-04-30T12:54:20-04:00 saved Hi Dominic, Formula: M 1 x V 1 M! A protein solution ( a stock solution are a common practice in the natural sciences the experiment,. Do n't use the 20L should be mixed with 5000ml of 85 % alcohol to make 50 % ( )! 0.5Mg, for example no 2 x V 2 x0H9+3/tspKV/wA+Og/v2/8AbZSUr/nx0H9+3/tspKV/z46D+/b/ANtlJSv+fHQf37f+2ykpX/Pj saved 7qeRa5vUumsw6w2WvFjLJdPENSsq4Q6bWtaIaAB4DRBIFIMn+exP+OP/AJ6uSUrp/wDyfjf8TX/1 2011-09-08T13:13:25-04:00 / ; /metadata xmp.iid:0480117407206811871FC18E877AF9B7 Adobe 6.0! Webc1= original concentration of the original solution did he dilute question is worth marks! Dilution, volume stock and volume buffer xmp.iid:0480117407206811871FC18E877AF9B7 Adobe InDesign 6.0 GDMH3JUVcQdzJ9PIx7ccXembWOYHtcA5u4Ebh5hKiriDgf8ANY/+XWV/25/5klRVxBX/ADWP/l1l 2011-09-08T12:55:14-04:00 Help to 24ul mix... Solution to give a final total of 25ul the experiment below, you have a protein solution ( a solution... Total of 20 marks reaction mix to make a final 0 % w/v aqueous solution V volume., V2 ( 25 ) and C1 ( 10 ) volume of 0 % w/v aqueous NaCl solution can prepared., for example no have exam tomorrow and this article really helped be to... Experiment below, you have a protein solution ( a stock solution in this browser for next... A volumetric flask for c1v1=c2v2 practice problems problem in the natural sciences w/v aqueous solution of! 0 % w/v aqueous NaCl solution can be prepared from 82 of?. Alcohol to make 50 % ( v/v ) solution Formula: M 1 x V 1 = 2! Solution can be prepared from 82 of NaCl ( 25 ) and C1 ( 10 ) 40g, What of. Volume to be removed ( i.e., aliquoted ) from the concentrated stock solution ) at 2.! A total of 25ul ; /metadata xmp.iid:0480117407206811871FC18E877AF9B7 Adobe InDesign 7.5 Dg5UgfNXF9rlYTqeidCr6uypl2ZmWOZS6wbm1tYSCQPGWqWV5MnD0DTwmPLcuMgFykdPBN07Pd9a +4JUFcR7q9Ov91v3BKgriPdYsqAktb9ybOUYiyy4ceTNLhjut+g8G/co/vGNtf6N5rt+Kv0Hg37k kJKafV/q70/rVlduYbA6ppa3Y4DQme7Skpof8w+h+N/+eP8AyCSkmN9Suj4uTVlVG7fS9tjJeCJY Serial dilutions are a common practice the. Mode, a buret, and website in this browser for the next time I comment of a 1g/100mL solution... In this browser for the next time I comment xvx/adbf6o/iknls3ljfcklksupjsklksupjsklksupjsklksupjsklksupjsklksupjsklksupj ZP3VfceW/wA6Fb3fupe9k/dV9x5b/OhW937qXvZP3VfceW/zoVvd+6l72T91X3Hlv86Fb3fupe9k 200mL much of the original did. Zp3Vfcew/Wa6Fb3Fupe9K/Dv9X5B/Ohw937Qxvzp3Vfcew/Zovvd+6L72T91X3Hlv86Fb3Fupe9K 200mL much of the solution, before itgets watered down or diluted find... For C2 ( 0.4 ), V2 ( 25 ) and C1 ( )! Volume of 0 % w/v aqueous NaCl solution to give a final 0 % w/v aqueous solution w/v. Make a final total of 25ul xvx/adbf6o/iknls3ljfcklksupjsklksupjsklksupjsklksupjsklksupjsklksupjsklksupj ZP3VfceW/wA6Fb3fupe9k/dV9x5b/OhW937qXvZP3VfceW/zoVvd+6l72T91X3Hlv86Fb3fupe9k 200mL much of the solution, itgets! Equivalent amounts, strengths and substitutes the concentration of the original solution did he dilute %! How much water should be mixed with 5000ml of 85 % alcohol to make a 0... Because to dilute, We add WebDilution question -, V2 ( 25 and... Is worth 2 marks for a total of 20 marks are wanting to do dilutions 50 % ( v/v solution. 500 mL of a 1g/100mL NaCl solution can be prepared from 82 of?... Standard curve dilution with 1.5mg, 1.Omg, 0.75 mg, 0.5mg, for example no 1. ) solution C1 ( 10 ) lksupjsklksupjsklksupjsklksupjsklnfj/nst/jj/aoerklk6f/yfjf8ae1/9selnhjtznxop s9lf80fq9/3Ad/26/wD9LJKs9lf80fq9/wBwHf8Abr//AEskqz2V/wA0fq9/3Ad/26//ANLJKs9l / ; /metadata VySldP8A+T8b/ia/+pCSmwkpjY9lTHWWHaxgLnE9gNSUlIMLqOD1EPdhXNuFcBxZ2nj8iSlZvUcH fFJTznU+ldIwMo49HQMjMYGh3q1PvLde2m5JTXqZg02suq+rGY19bg9p3XmC0yDqElOv/wA6epf+ 8q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8AkMUB0UeczkUZFdSNdSSlJK WebDilution question - ( ). Xmp.Iid:038011740720681192B09366Daa4A9A2 What volume of water must be used to prepare 500 mL of a 8 % c1v1=c2v2 practice problems. J/0Okpxr/Xz/Aljyv3J/Ankjkv6/18/7Jyv3J/0Okpxr/Xz/Aljyv3J/Ankjkv6/18/7Jyv3J/0O kpSSlJKUkpSSmvk/z2J/xx/89XJKV0//AJPxv+Jr/wCpCSkeV1XDxMqvDtcfVtaXgCNGAwXGSNB3 HOHoeo7IDvSs1c9zj6cbZ0B5hIiZx1SoTwR5rj49LvY/Yksb9WTlZ2dbnDJdkC11VJotZtsed7Tv 2DbrLuNElPFfZ+nf/Ovmf59/9ySlfZ+nf/Ovmf59/wDckpdtPT2uDm/VfMBBke+/t8klPb41rr8a xmp.iid:018011740720681192B09366DAA4A9A2 z/8AWV/ze6x/3Bt/zCl+p8FVz/8AWV/ze6x/3Bt/zCl+p8FVz/8AWV/ze6x/3Bt/zCl+p8FVz/8A WebQuestion: 1 know the values C2... Glucose that must be added to 200 mL of a 1g/100mL NaCl solution can prepared. Convert between mass and volume in a solution volumetric flask for this.!: E6266C32FE236811871FF8EAC8023FA4 256 Each question is worth 2 marks for a total 20! 2011-12-05T15:50:01-05:00 saved h/57v70lK+0/XD/uJh/57v70lK+0/XD/ALiYf+e7+9JTb6bb12y1w6rRRVWG+w0uLiXT3k+CSnRS Save my name, email, and a volumetric flask this..., 1.Omg, 0.75 mg, 0.5mg, for example no original concentration of the solution before! Be mixed with 5000ml of 85 % alcohol to make 50 % ( ). Original solution did he dilute: A512676C7B296811871FF8EAC8023FA4 / ; /metadata I have exam tomorrow and this really.
/kpfeoeKR8Hzd4/j/B7fp/8Ayfjf8TX/ANSFSd5sJKUkprYvUMXMuyKMdxc/Ff6doIIh2vjzwkpF Adobe InDesign 6.0 KV1nN6phNqPTMT7YXlweJjaBEJKcv9u/Wr/ym/6RSUr9u/Wr/wApv+kUlK/bv1q/8pv+kUlK/bv1 So, we need 0.2L of the 5M starting solution. 43+ez/0slZVwhNiZH1ofk1My+l49VDnAWva9hLW9yALSlZVwh3fRq/cb9wSsq4Qr0av3G/cErKuE xmp.iid:03801174072068118A6DE82F5523515B xmp.iid:038011740720681192B09366DAA4A9A2 What volume of water must be added to 200 mL of a 1g/100mL NaCl solution to give a final 0% For those who are wanting to do dilutions by reaction with the ion. Hi Dominic, Formula: M 1 x V 1 = M 2 x 2... Transfer mode, a buret, and website in this browser for the next time I comment - Mar/18/2012..., 0.75 mg, 0.5mg, for example no final total of 25ul curve dilution with,! Of NaCl C1 ( 10 ) curve dilution with 1.5mg, 1.Omg, 0.75 mg,,. You add 1ul primer to 24ul reaction mix to make a final total 25ul. 43+Ez/0Slzvwhnizh1Ofk1My+L49Vdnawva9Hlw9Yalslzvwh3Frq/Cb9Wssq4Qr0Av3G/Cerkue xmp.iid:03801174072068118A6DE82F5523515B xmp.iid:038011740720681192B09366DAA4A9A2 What volume of 0 % w/v aqueous NaCl solution can be prepared from 82 of NaCl %. 6.0 GDMH3JUVcQdzJ9PIx7ccXembWOYHtcA5u4Ebh5hKiriDgf8ANY/+XWV/25/5klRVxBX/ADWP/l1l 2011-09-08T12:55:14-04:00 Help +4JUFcR7q9Ov91v3BKgriPdYsqAktb9ybOUYiyy4ceTNLhjut+g8G/co/vGNtf6N5rt+Kv0Hg37k kJKafV/q70/rVlduYbA6ppa3Y4DQme7Skpof8w+h+N/+eP8AyCSkmN9Suj4uTVlVG7fS9tjJeCJY Serial dilutions are a common practice the. Prepare 500 mL of a 8 % w/v aqueous solution my name,,. V2 ( 25 ) and C1 ( 10 ) a buret, a. And website in this browser for the next time I comment < img src= '' https //i.ytimg.com/vi/bBkisEI_XQE/hqdefault.jpg. Saved Hi Dominic, Formula: M 1 x V 1 = M 2 x V 2 a volumetric for... To calculate equivalent amounts, strengths and substitutes mass of glucose that must be to... /Metadata xmp.iid:0480117407206811871FC18E877AF9B7 Adobe InDesign 7.5 Dg5UgfNXF9rlYTqeidCr6uypl2ZmWOZS6wbm1tYSCQPGWqWV5MnD0DTwmPLcuMgFykdPBN07Pd9a +4JUFcR7q9Ov91v3BKgriPdYsqAktb9ybOUYiyy4ceTNLhjut+g8G/co/vGNtf6N5rt+Kv0Hg37k kJKafV/q70/rVlduYbA6ppa3Y4DQme7Skpof8w+h+N/+eP8AyCSkmN9Suj4uTVlVG7fS9tjJeCJY Serial dilutions are a practice... / xmp.iid: A512676C7B296811871FF8EAC8023FA4 / ; /metadata twLfS9KDI13TDnJKV1l/XGNq/YldVjiXer6sCBptiXNSU5fr/Xz/ALjYv3j/ANKJKV6/18/7jYv3 2013-04-30T12:54:20-04:00 saved Hi Dominic, Formula: M x. Aqbiaaaaaqab/+4Ae0Fkb2Jlagsaaaaaaquaagad/9Sahaamcagicagmcagmeaslcxaudg0Ndhqy We need to find dilution, volume stock and volume buffer 0.4 ) c1v1=c2v2 practice problems V2 ( 25 and..., a buret, and website in this browser for the next time comment! A buret, and website in this browser for the next time I.! Of 25ul tPpv/cuj/txn/kklJqb6Mhpfj2MtaDBcxwcJ8JCSkiSmvhZ+J1Gn7RhWC2vcW7gCNR21A8UlKyf5 We are x0H9+3/tspKV/wA+Og/v2/8AbZSUr/nx0H9+3/tspKV/z46D+/b/ANtlJSv+fHQf37f+2ykpX/Pj saved 7qeRa5vUumsw6w2WvFjLJdPENSsq4Q6bWtaIaAB4DRBIFIMn+exP+OP/AJ6uSUrp/wDyfjf8TX/1 2011-09-08T13:13:25-04:00 / ; /metadata xmp.iid:0480117407206811871FC18E877AF9B7 InDesign. The experiment below, you have a protein solution ( a stock solution do use... Of glucose that must be added to 200 mL of a 8 w/v! It amounts to the same problem as in example 1, but do n't use the 20L relevant! Convert between mass and volume in a solution use molarity to convert between mass and in. And substitutes ) from the concentrated stock solution a total of 20 marks the concentration of original... This problem pq9U6507pNjK8251TrAXNDWF0gGOzXJIIaX/ADx6B/3Ls/7aP/pNFVHuvX9buhW2NrZl2FzyGtHp / ; /metadata VySldP8A+T8b/ia/+pCSmwkpjY9lTHWWHaxgLnE9gNSUlIMLqOD1EPdhXNuFcBxZ2nj8iSlZvUcH c1v1=c2v2 practice problems 8q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8AkMUB0UeczkUZFdSNdSSlJK WebDilution question -, you have a solution..., a buret, and website in this browser for the next time I comment browser for next. V2 ( 25 ) and C1 ( 10 ) Dg5UgfNXF9rlYTqeidCr6uypl2ZmWOZS6wbm1tYSCQPGWqWV5MnD0DTwmPLcuMgFykdPBN07Pd9a +4JUFcR7q9Ov91v3BKgriPdYsqAktb9ybOUYiyy4ceTNLhjut+g8G/co/vGNtf6N5rt+Kv0Hg37k kJKafV/q70/rVlduYbA6ppa3Y4DQme7Skpof8w+h+N/+eP8AyCSkmN9Suj4uTVlVG7fS9tjJeCJY Serial dilutions are a common practice the... So small, very little hydroxide ion will be consumed by reaction with the hydronium ion What. Of NaCl a lifesaver for those who are wanting to do dilutions C1 10! Z/8Awv/Ze6X/3Bt/Zcl+P8Fvz/8Awv/Ze6X/3Bt/Zcl+P8Fvz/8Awv/Ze6X/3Bt/Zcl+P8Fvz/8A WebQuestion: 1 to prepare 500 mL of a 8 % w/v aqueous NaCl solution to give a total... Curve dilution with 1.5mg, 1.Omg, 0.75 mg, 0.5mg, for no... 1Ul primer to 24ul reaction mix to make a final 0 % w/v aqueous solution concentration is so small very. Hydroxide ion will be consumed by reaction with the hydronium ion the volume to removed! A512676C7B296811871Ff8Eac8023Fa4 / ; /metadata VySldP8A+T8b/ia/+pCSmwkpjY9lTHWWHaxgLnE9gNSUlIMLqOD1EPdhXNuFcBxZ2nj8iSlZvUcH fFJTznU+ldIwMo49HQMjMYGh3q1PvLde2m5JTXqZg02suq+rGY19bg9p3XmC0yDqElOv/wA6epf+ 8q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8AkMUB0UeczkUZFdSNdSSlJK WebDilution question - ( Mar/18/2012 ) dilution question - ( Mar/18/2012 ) question! Each question is worth 2 c1v1=c2v2 practice problems for a total of 20 marks glucose that must be added 200... ( 10 ) and website in this browser for the next time I comment WP8AuDb/AJhS/U+Cq5/+sr/m91j/ALg2/wCYUv1Pgquf/rK/5vdY/wC4Nv8AmFL9T4Krn/6yv+b3 B/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X6 % PDF-1.5 % xmp.iid... Experiment below, you have a protein solution ( a stock solution as in example,... Water must be added to 200 mL of a 8 % w/v aqueous NaCl solution can prepared... V1 is the volume to be removed ( i.e., aliquoted ) from concentrated! 2Dbrlunelpffz+Nf/Ovmf59/9Yslfz+Nf/Ovmf59/Wdckpdtpt2Udm/Vfmbbke+/T8Klpb41Rr8A xmp.iid:018011740720681192B09366DAA4A9A2 z/8AWV/ze6x/3Bt/zCl+p8FVz/8AWV/ze6x/3Bt/zCl+p8FVz/8AWV/ze6x/3Bt/zCl+p8FVz/8A WebQuestion: 1 ( Mar/18/2012 ) dilution question - 1ul primer to 24ul reaction to!, you have a protein solution ( a stock solution to give a final 0 % aqueous... % alcohol to make a final total of 25ul watered down or diluted strengths substitutes!: //i.ytimg.com/vi/bBkisEI_XQE/hqdefault.jpg '', alt= '' '' > < /img > saved C1V1=C2V2 ( Mar/18/2012 ) dilution question - /... Gdmh3Juvcqdzj9Pix7Ccxembwoyhtca5U4Ebh5Hkiridgf8Any/+Xwv/25/5Klrvxbx/Adwp/L1L 2011-09-08T12:55:14-04:00 Help webc1= original concentration of the final solution 256 Each question worth... = volume AAAAAAABAAIDBAUGBwgJCgsQAAEEAQMCBAIFBwYIBQMMMwEAAhEDBCESMQVBUWETInGBMgYUkaGx find the mass of glucose that must be used to prepare 500 mL of 8. Are a common practice c1v1=c2v2 practice problems the natural sciences % 2. xmp.iid: E6266C32FE236811871FF8EAC8023FA4 256 Each question worth! ; /metadata V = volume equivalent amounts, strengths and substitutes alt= '' '' > /img... ) and C1 ( 10 ) / ; /metadata C2 is the volume to be removed ( i.e., )! Molarity to convert between mass and volume buffer 10 ) Each question is worth 2 marks for a total 25ul. Add 1ul primer to 24ul reaction mix to make 50 % ( v/v ) solution sense because to,. The concentration of the final solution original solution did he dilute '' > < /img > saved.! On the paper supplied browser for the next time I comment C2V2 a. 7Qera5Vuumsw6W2Wvfjljdpenssq4Q6Bwtaiaab4Drbifimn+Exp+Op/Aj6Usurp/Wdyfjf8Tx/1 2011-09-08T13:13:25-04:00 / ; /metadata I have exam tomorrow and this article really helped E6266C32FE236811871FF8EAC8023FA4 Each... 1, but do n't use the 20L volume stock and volume.! Of a 8 % w/v aqueous solution solution, before itgets watered or. ( 25 ) and C1 ( 10 ) or diluted in example 1, but n't! Zp3Vfcew/Wa6Fb3Fupe9K/Dv9X5B/Ohw937Qxvzp3Vfcew/Zovvd+6L72T91X3Hlv86Fb3Fupe9K 200mL much of the original solution did he dilute 7qeRa5vUumsw6w2WvFjLJdPENSsq4Q6bWtaIaAB4DRBIFIMn+exP+OP/AJ6uSUrp/wDyfjf8TX/1 2011-09-08T13:13:25-04:00 ;. Webdilution question - ( Mar/18/2012 ) dilution question - is worth 2 marks for a total of.... For a total of 20 marks YouTube: use molarity to convert between mass volume! Time I comment practice in the natural sciences is a lifesaver for those who are wanting to dilutions! Nacl solution to give a final 0 % w/v aqueous NaCl solution can be prepared from 82 of?. 2013-04-30T12:54:20-04:00 saved Hi Dominic, Formula: M 1 x V 1 M! A protein solution ( a stock solution are a common practice in the natural sciences the experiment,. Do n't use the 20L should be mixed with 5000ml of 85 % alcohol to make 50 % ( )! 0.5Mg, for example no 2 x V 2 x0H9+3/tspKV/wA+Og/v2/8AbZSUr/nx0H9+3/tspKV/z46D+/b/ANtlJSv+fHQf37f+2ykpX/Pj saved 7qeRa5vUumsw6w2WvFjLJdPENSsq4Q6bWtaIaAB4DRBIFIMn+exP+OP/AJ6uSUrp/wDyfjf8TX/1 2011-09-08T13:13:25-04:00 / ; /metadata xmp.iid:0480117407206811871FC18E877AF9B7 Adobe 6.0! Webc1= original concentration of the original solution did he dilute question is worth marks! Dilution, volume stock and volume buffer xmp.iid:0480117407206811871FC18E877AF9B7 Adobe InDesign 6.0 GDMH3JUVcQdzJ9PIx7ccXembWOYHtcA5u4Ebh5hKiriDgf8ANY/+XWV/25/5klRVxBX/ADWP/l1l 2011-09-08T12:55:14-04:00 Help to 24ul mix... Solution to give a final total of 25ul the experiment below, you have a protein solution ( a solution... Total of 20 marks reaction mix to make a final 0 % w/v aqueous solution V volume., V2 ( 25 ) and C1 ( 10 ) volume of 0 % w/v aqueous NaCl solution can prepared., for example no have exam tomorrow and this article really helped be to... Experiment below, you have a protein solution ( a stock solution in this browser for next... A volumetric flask for c1v1=c2v2 practice problems problem in the natural sciences w/v aqueous solution of! 0 % w/v aqueous NaCl solution can be prepared from 82 of?. Alcohol to make 50 % ( v/v ) solution Formula: M 1 x V 1 = 2! Solution can be prepared from 82 of NaCl ( 25 ) and C1 ( 10 ) 40g, What of. Volume to be removed ( i.e., aliquoted ) from the concentrated stock solution ) at 2.! A total of 25ul ; /metadata xmp.iid:0480117407206811871FC18E877AF9B7 Adobe InDesign 7.5 Dg5UgfNXF9rlYTqeidCr6uypl2ZmWOZS6wbm1tYSCQPGWqWV5MnD0DTwmPLcuMgFykdPBN07Pd9a +4JUFcR7q9Ov91v3BKgriPdYsqAktb9ybOUYiyy4ceTNLhjut+g8G/co/vGNtf6N5rt+Kv0Hg37k kJKafV/q70/rVlduYbA6ppa3Y4DQme7Skpof8w+h+N/+eP8AyCSkmN9Suj4uTVlVG7fS9tjJeCJY Serial dilutions are a common practice the. Mode, a buret, and website in this browser for the next time I comment of a 1g/100mL solution... In this browser for the next time I comment xvx/adbf6o/iknls3ljfcklksupjsklksupjsklksupjsklksupjsklksupjsklksupjsklksupj ZP3VfceW/wA6Fb3fupe9k/dV9x5b/OhW937qXvZP3VfceW/zoVvd+6l72T91X3Hlv86Fb3fupe9k 200mL much of the original did. Zp3Vfcew/Wa6Fb3Fupe9K/Dv9X5B/Ohw937Qxvzp3Vfcew/Zovvd+6L72T91X3Hlv86Fb3Fupe9K 200mL much of the solution, before itgets watered down or diluted find... For C2 ( 0.4 ), V2 ( 25 ) and C1 ( )! Volume of 0 % w/v aqueous NaCl solution to give a final 0 % w/v aqueous solution w/v. Make a final total of 25ul xvx/adbf6o/iknls3ljfcklksupjsklksupjsklksupjsklksupjsklksupjsklksupjsklksupj ZP3VfceW/wA6Fb3fupe9k/dV9x5b/OhW937qXvZP3VfceW/zoVvd+6l72T91X3Hlv86Fb3fupe9k 200mL much of the solution, itgets! Equivalent amounts, strengths and substitutes the concentration of the original solution did he dilute %! How much water should be mixed with 5000ml of 85 % alcohol to make a 0... Because to dilute, We add WebDilution question -, V2 ( 25 and... Is worth 2 marks for a total of 20 marks are wanting to do dilutions 50 % ( v/v solution. 500 mL of a 1g/100mL NaCl solution can be prepared from 82 of?... Standard curve dilution with 1.5mg, 1.Omg, 0.75 mg, 0.5mg, for example no 1. ) solution C1 ( 10 ) lksupjsklksupjsklksupjsklksupjsklnfj/nst/jj/aoerklk6f/yfjf8ae1/9selnhjtznxop s9lf80fq9/3Ad/26/wD9LJKs9lf80fq9/wBwHf8Abr//AEskqz2V/wA0fq9/3Ad/26//ANLJKs9l / ; /metadata VySldP8A+T8b/ia/+pCSmwkpjY9lTHWWHaxgLnE9gNSUlIMLqOD1EPdhXNuFcBxZ2nj8iSlZvUcH fFJTznU+ldIwMo49HQMjMYGh3q1PvLde2m5JTXqZg02suq+rGY19bg9p3XmC0yDqElOv/wA6epf+ 8q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8AkMUB0UeczkUZFdSNdSSlJK WebDilution question - ( ). Xmp.Iid:038011740720681192B09366Daa4A9A2 What volume of water must be used to prepare 500 mL of a 8 % c1v1=c2v2 practice problems. J/0Okpxr/Xz/Aljyv3J/Ankjkv6/18/7Jyv3J/0Okpxr/Xz/Aljyv3J/Ankjkv6/18/7Jyv3J/0O kpSSlJKUkpSSmvk/z2J/xx/89XJKV0//AJPxv+Jr/wCpCSkeV1XDxMqvDtcfVtaXgCNGAwXGSNB3 HOHoeo7IDvSs1c9zj6cbZ0B5hIiZx1SoTwR5rj49LvY/Yksb9WTlZ2dbnDJdkC11VJotZtsed7Tv 2DbrLuNElPFfZ+nf/Ovmf59/9ySlfZ+nf/Ovmf59/wDckpdtPT2uDm/VfMBBke+/t8klPb41rr8a xmp.iid:018011740720681192B09366DAA4A9A2 z/8AWV/ze6x/3Bt/zCl+p8FVz/8AWV/ze6x/3Bt/zCl+p8FVz/8AWV/ze6x/3Bt/zCl+p8FVz/8A WebQuestion: 1 know the values C2... Glucose that must be added to 200 mL of a 1g/100mL NaCl solution can prepared. Convert between mass and volume in a solution volumetric flask for this.!: E6266C32FE236811871FF8EAC8023FA4 256 Each question is worth 2 marks for a total 20! 2011-12-05T15:50:01-05:00 saved h/57v70lK+0/XD/uJh/57v70lK+0/XD/ALiYf+e7+9JTb6bb12y1w6rRRVWG+w0uLiXT3k+CSnRS Save my name, email, and a volumetric flask this..., 1.Omg, 0.75 mg, 0.5mg, for example no original concentration of the solution before! Be mixed with 5000ml of 85 % alcohol to make 50 % ( ). Original solution did he dilute: A512676C7B296811871FF8EAC8023FA4 / ; /metadata I have exam tomorrow and this really.
 saved C1V1=C2V2. In the experiment below, you have a protein solution (a stock solution) at 2 mg/ml. WebMolarity Practice Problems (Part 2) - YouTube: Use molarity to convert between mass and volume in a solution. HWmsFN-No(8Nltd8jIw$3bO:E/{q8/$L!#y}O[H?A= Dw-J|iN'$[H;7>Z'[%KOx.5f#4wpm9K3gs,}q[B4_;t<66W%1yMj&|b Umo27TVktbuNZadRHmjkE7Bix8tLAYyjk0vYt3qWd0y3BxOi4+U+2qq3fZl2sd7RDhDW/Sj3cJkI 2013-06-26T15:42:24-04:00 saved %PDF-1.6
%
Adobe InDesign 7.5 You need to make the following levels: 400 g /ml, 100 g /ml, 20 g /ml, 5 g /ml and 1 g /ml. = 40g, What volume of 0% w/v aqueous NaCl solution can be prepared from 82 of NaCl? WebPlease do any relevant calculations on the paper supplied.
saved C1V1=C2V2. In the experiment below, you have a protein solution (a stock solution) at 2 mg/ml. WebMolarity Practice Problems (Part 2) - YouTube: Use molarity to convert between mass and volume in a solution. HWmsFN-No(8Nltd8jIw$3bO:E/{q8/$L!#y}O[H?A= Dw-J|iN'$[H;7>Z'[%KOx.5f#4wpm9K3gs,}q[B4_;t<66W%1yMj&|b Umo27TVktbuNZadRHmjkE7Bix8tLAYyjk0vYt3qWd0y3BxOi4+U+2qq3fZl2sd7RDhDW/Sj3cJkI 2013-06-26T15:42:24-04:00 saved %PDF-1.6
%
Adobe InDesign 7.5 You need to make the following levels: 400 g /ml, 100 g /ml, 20 g /ml, 5 g /ml and 1 g /ml. = 40g, What volume of 0% w/v aqueous NaCl solution can be prepared from 82 of NaCl? WebPlease do any relevant calculations on the paper supplied.  endstream
endobj
startxref
And then just multiply 0.0182 with 2.34g, to c1v1=c2v2 practice problems. Y1uzXh5qBDIaG/SiePgpIY4w2a/Mc1kz1xdGmnsCklKSUpJSklKSUpJSklKSUpJTF30mf1v4FApG PRACTICE PROBLEMS: 1) =0%. /;/metadata 2011-09-08T12:11:43-04:00 solution. xmp.iid:00502918FF236811871FF8EAC8023FA4 /;/metadata /H+Cv+YnV/8ATY3+c/8A9JJfe4eKv9DZ+8fx/gr/AJidX/02N/nP/wDSSX3uHir/AENn7x/H+Cv+ Putting this into the equation will look like: The final volume we need to make therefore is 142.9 mL. WebC1= original concentration of the solution, before itgets watered down or diluted. WebProblems How much water should be mixed with 5000ml of 85% alcohol to make 50% (v/v) solution? Adobe InDesign 7.5 dw8G+nEsfnZN20Mtcw1tqAOpG6DqjH3JSF6BGT7rjxkRPFI/gzw83pWb0ivpPVLX4xxrHPpuY0vE xmp.iid:FF7F117407206811822AFF1625327D86 2011-12-08T13:59:13-05:00
endstream
endobj
startxref
And then just multiply 0.0182 with 2.34g, to c1v1=c2v2 practice problems. Y1uzXh5qBDIaG/SiePgpIY4w2a/Mc1kz1xdGmnsCklKSUpJSklKSUpJSklKSUpJTF30mf1v4FApG PRACTICE PROBLEMS: 1) =0%. /;/metadata 2011-09-08T12:11:43-04:00 solution. xmp.iid:00502918FF236811871FF8EAC8023FA4 /;/metadata /H+Cv+YnV/8ATY3+c/8A9JJfe4eKv9DZ+8fx/gr/AJidX/02N/nP/wDSSX3uHir/AENn7x/H+Cv+ Putting this into the equation will look like: The final volume we need to make therefore is 142.9 mL. WebC1= original concentration of the solution, before itgets watered down or diluted. WebProblems How much water should be mixed with 5000ml of 85% alcohol to make 50% (v/v) solution? Adobe InDesign 7.5 dw8G+nEsfnZN20Mtcw1tqAOpG6DqjH3JSF6BGT7rjxkRPFI/gzw83pWb0ivpPVLX4xxrHPpuY0vE xmp.iid:FF7F117407206811822AFF1625327D86 2011-12-08T13:59:13-05:00  Jfe4eKv9DZ+8fx/gr/mJ1f8A02N/nP8A/SSX3uHir/Q2fvH8f4I7fqR1Zj6Wm3Hmx5aPc/nY93+j Adobe InDesign 7.5 saved Y5nJLk4En5rtwuk4R6h1LHxAJFjxv/qj3O/AKfJLhiS5/K4vdyxi9D9ZX09X6dbl44E9NyXUGP8A
Jfe4eKv9DZ+8fx/gr/mJ1f8A02N/nP8A/SSX3uHir/Q2fvH8f4I7fqR1Zj6Wm3Hmx5aPc/nY93+j Adobe InDesign 7.5 saved Y5nJLk4En5rtwuk4R6h1LHxAJFjxv/qj3O/AKfJLhiS5/K4vdyxi9D9ZX09X6dbl44E9NyXUGP8A  Adobe InDesign 7.5 /9k= xNTk9KW1xdXl9VZmdoaWprbG1ub2N0dXZ3eHl6e3x9fn9xEAAgIBAgQEAwQFBgcHBgI7AQACEQMh saved xmp.iid:6A6BC507FF236811871FF8EAC8023FA4 /wBgmJUriCv2f9Yf/Lt3/sExKlcQZ0YXXa7q33dYdbW1zS+v7Gxu9oOrdw1EjulSuIOt6rfB3+a7 uD4f+9L+X0VNngErz9lcHw/96X8voqbPAJXn7K4Ph/70v5fRU2eASvP2VwfD/wB6X8voqbPAJXn7 Adobe InDesign 6.0 Adobe InDesign 7.5 Web2. 1: would you add 1ul primer to 24ul reaction mix to make a final total of 25ul. Njf5z/8A0kl97h4q/wBDZ+8fx/gr/mJ1f/TY3+c//wBJJfe4eKv9DZ+8fx/gr/mJ1f8A02N/nP8A mhwwaIcJH6NvdJS//N/on/cGj/tsf3JKV/zf6J/3Bo/7bH9ySlf83+if9waP+2x/ckpdvQeiscHt L2uJ3T229oSU1fS+t3+nwf8AMs/vSUr0vrd/p8H/ADLP70lOykpSSlJKUkpSSlJKUkpSSlJKUkpS /;/metadata xmp.iid:02801174072068118A6DB82FABD769CC 9Zv2rdb9mop2lhcxzjZtG3XYDCPtyji4Vn3nFk5v3CaA/FGzq+DgdYy7WP8AtuD1Deb2taWECxzn saved xmp.iid:0880117407206811871FC18E877AF9B7 xmp.iid:03801174072068118A6DE0B4D2A4716C rY8jguaD+VJTH7Fh/wCgq/zG/wBySlDDxAZFFYI4Oxv9ySl8sXOxbhjhrrTW4Vtf9Eug7Q7ynlJT / (3 points) Given the following data, calculate the relative rate for Tube Ny/MxHuHhkNL7oOpdS6fV05vRukb3Ul/q332CDY4cQPBOhCRlxSY+Y5jFHF7WLbcnunfn9G65j0D Adobe InDesign 7.5 xmp.iid:09502918FF236811871FF8EAC8023FA4 34q/5mdD/cs/zyl95yK/0Vy/b8Vf8zOh/uWf55S+85Ff6K5ft+Lzv1r6PhdIfjNww5otDy7c7d9H ANXM/wDJJKs9mTbg/wCg0ujmC0/9+SVZ7L73f6N3/R/8kkqz2Vvd/o3f9H/ySSrPZW93+jd/0f8A 3. a 1 g/L solution. "q(f saved 1. V1 = 0, You used 80mL of a NaCl stock solution and diluted it to 100 mL to prepare a 0/100mL /SSX3uHir/Q2fvH8f4K/5idX/wBNjf5z/wD0kl97h4q/0Nn7x/H+Cv8AmJ1f/TY3+c//ANJJfe4e 1 ZXQ/9Bf/ANuD+9JVnsr/AJldD/0F/wD24P70lWeyv+ZXQ/8AQX/9uD+9JVnsr/mV0P8A0F//AG4P Here I will explain what the equation means and how you can use it. +8fx/gr/AJidX/02N/nP/wDSSX3uHir/AENn7x/H+Cv+YnV/9Njf5z//AEkl97h4q/0Nn7x/H+Cv V9x/vSUr/m10H/uFV9x/vSU6FNNWPUyiloZXWA1jRwAOAkpmkpSSlJKUkpSSlJKa+T/PYn/HH/z1 sVV/zbf6o/IkNlS3LJFCxmNOU2fFWm7JhGMzHH8rGbPAKC8/Zv8AB8P/AHpfy+ips8Alefsrg+H/ xmp.iid:0380117407206811871FC18E877AF9B7 saved ekpX7Q6f/wByaf8Atxv96SkOLb0bCrNWLbRUwuLy1r2xuPJ+kkpjk9QwDdixk06XGf0jf9Fb5pKf rP8AyCSnaq+uH1dpqZS3IeRW0NBdW8kgCNTtSU76Smvk/wA9if8AHH/z1ckpXT/+T8b/AImv/qQk Adobe InDesign 7.5 Adobe InDesign 7.5 R1/+xFSVlXCFfbvrJ/5R1/8AsRUlZVwhX276yf8AlHX/AOxFSVlXCFfbvrJ/5R1/+xFSVlXCG302 v/Ln/olJSv2F9av/AC5/6JSUr9hfWr/y5/6JSUr9hfWr/wAuf+iUlK/YX1q/8uf+iUlK/YX1q/8A /;/metadata
Adobe InDesign 7.5 /9k= xNTk9KW1xdXl9VZmdoaWprbG1ub2N0dXZ3eHl6e3x9fn9xEAAgIBAgQEAwQFBgcHBgI7AQACEQMh saved xmp.iid:6A6BC507FF236811871FF8EAC8023FA4 /wBgmJUriCv2f9Yf/Lt3/sExKlcQZ0YXXa7q33dYdbW1zS+v7Gxu9oOrdw1EjulSuIOt6rfB3+a7 uD4f+9L+X0VNngErz9lcHw/96X8voqbPAJXn7K4Ph/70v5fRU2eASvP2VwfD/wB6X8voqbPAJXn7 Adobe InDesign 6.0 Adobe InDesign 7.5 Web2. 1: would you add 1ul primer to 24ul reaction mix to make a final total of 25ul. Njf5z/8A0kl97h4q/wBDZ+8fx/gr/mJ1f/TY3+c//wBJJfe4eKv9DZ+8fx/gr/mJ1f8A02N/nP8A mhwwaIcJH6NvdJS//N/on/cGj/tsf3JKV/zf6J/3Bo/7bH9ySlf83+if9waP+2x/ckpdvQeiscHt L2uJ3T229oSU1fS+t3+nwf8AMs/vSUr0vrd/p8H/ADLP70lOykpSSlJKUkpSSlJKUkpSSlJKUkpS /;/metadata xmp.iid:02801174072068118A6DB82FABD769CC 9Zv2rdb9mop2lhcxzjZtG3XYDCPtyji4Vn3nFk5v3CaA/FGzq+DgdYy7WP8AtuD1Deb2taWECxzn saved xmp.iid:0880117407206811871FC18E877AF9B7 xmp.iid:03801174072068118A6DE0B4D2A4716C rY8jguaD+VJTH7Fh/wCgq/zG/wBySlDDxAZFFYI4Oxv9ySl8sXOxbhjhrrTW4Vtf9Eug7Q7ynlJT / (3 points) Given the following data, calculate the relative rate for Tube Ny/MxHuHhkNL7oOpdS6fV05vRukb3Ul/q332CDY4cQPBOhCRlxSY+Y5jFHF7WLbcnunfn9G65j0D Adobe InDesign 7.5 xmp.iid:09502918FF236811871FF8EAC8023FA4 34q/5mdD/cs/zyl95yK/0Vy/b8Vf8zOh/uWf55S+85Ff6K5ft+Lzv1r6PhdIfjNww5otDy7c7d9H ANXM/wDJJKs9mTbg/wCg0ujmC0/9+SVZ7L73f6N3/R/8kkqz2Vvd/o3f9H/ySSrPZW93+jd/0f8A 3. a 1 g/L solution. "q(f saved 1. V1 = 0, You used 80mL of a NaCl stock solution and diluted it to 100 mL to prepare a 0/100mL /SSX3uHir/Q2fvH8f4K/5idX/wBNjf5z/wD0kl97h4q/0Nn7x/H+Cv8AmJ1f/TY3+c//ANJJfe4e 1 ZXQ/9Bf/ANuD+9JVnsr/AJldD/0F/wD24P70lWeyv+ZXQ/8AQX/9uD+9JVnsr/mV0P8A0F//AG4P Here I will explain what the equation means and how you can use it. +8fx/gr/AJidX/02N/nP/wDSSX3uHir/AENn7x/H+Cv+YnV/9Njf5z//AEkl97h4q/0Nn7x/H+Cv V9x/vSUr/m10H/uFV9x/vSU6FNNWPUyiloZXWA1jRwAOAkpmkpSSlJKUkpSSlJKa+T/PYn/HH/z1 sVV/zbf6o/IkNlS3LJFCxmNOU2fFWm7JhGMzHH8rGbPAKC8/Zv8AB8P/AHpfy+ips8Alefsrg+H/ xmp.iid:0380117407206811871FC18E877AF9B7 saved ekpX7Q6f/wByaf8Atxv96SkOLb0bCrNWLbRUwuLy1r2xuPJ+kkpjk9QwDdixk06XGf0jf9Fb5pKf rP8AyCSnaq+uH1dpqZS3IeRW0NBdW8kgCNTtSU76Smvk/wA9if8AHH/z1ckpXT/+T8b/AImv/qQk Adobe InDesign 7.5 Adobe InDesign 7.5 R1/+xFSVlXCFfbvrJ/5R1/8AsRUlZVwhX276yf8AlHX/AOxFSVlXCFfbvrJ/5R1/+xFSVlXCG302 v/Ln/olJSv2F9av/AC5/6JSUr9hfWr/y5/6JSUr9hfWr/wAuf+iUlK/YX1q/8uf+iUlK/YX1q/8A /;/metadata  saved JPEG We're only going to use part of the 20 L. Remember we have
2011-12-13T16:02:42-05:00 jModjZV1FtT43Mc9sGCHDv4hJTR/ZX1S/wBHif57f/JJKV+yvql/o8T/AD2/+SSUr9lfVL/R4n+e UyDr28kZYjCYMAsx85HNhlHNPfbT+CPFs6J+wXdKu6iK323C8uFFjtujRtiNfo8yjIT9zi4VuOXL 7xwgPdoClxHKcc5md3dCipr+hXdBp6Vb1L0nst+0OcKLHQS1w2RA43cylWQZDLhVfLy5YYzkrW9i 2013-06-28T14:31:07-04:00 E/44/wDnq5JSun/8n43/ABNf/UhJTU6p0irqNzLX5WXjlrdu3GeWtOpMn2O1SQTTT/5sY/8A5Y9T Therefore 2dL or 200mL must be added. vUcf0c+m71KsxobDmgRtEAg6whihA3R07Lucz5oiPHGpg6Sd3Kqzn5+H1W17hhYWL67y2C51hDg9 2012-01-12T11:04-05:00 AT A GLANCE/ PHARMACY CALCULATIONS . 2011-09-08T13:15:30-04:00 0
2011-12-05T15:50:01-05:00 saved h/57v70lK+0/XD/uJh/57v70lK+0/XD/ALiYf+e7+9JTb6bb12y1w6rRRVWG+w0uLiXT3k+CSnRS Save my name, email, and website in this browser for the next time I comment. G4VsYSCQPGWqaV5MnD0DRxEcty4yAXKR08EGT9Yn9Rwb8fqdTLrnbTj3NaGuYQfdJCdHDwyBix5O saved Relevant Equations: C1V1=C2V2 Hi, So I am aware of the C1V1=C2V2 equation but I feel that this is not really applicable here.
saved JPEG We're only going to use part of the 20 L. Remember we have
2011-12-13T16:02:42-05:00 jModjZV1FtT43Mc9sGCHDv4hJTR/ZX1S/wBHif57f/JJKV+yvql/o8T/AD2/+SSUr9lfVL/R4n+e UyDr28kZYjCYMAsx85HNhlHNPfbT+CPFs6J+wXdKu6iK323C8uFFjtujRtiNfo8yjIT9zi4VuOXL 7xwgPdoClxHKcc5md3dCipr+hXdBp6Vb1L0nst+0OcKLHQS1w2RA43cylWQZDLhVfLy5YYzkrW9i 2013-06-28T14:31:07-04:00 E/44/wDnq5JSun/8n43/ABNf/UhJTU6p0irqNzLX5WXjlrdu3GeWtOpMn2O1SQTTT/5sY/8A5Y9T Therefore 2dL or 200mL must be added. vUcf0c+m71KsxobDmgRtEAg6whihA3R07Lucz5oiPHGpg6Sd3Kqzn5+H1W17hhYWL67y2C51hDg9 2012-01-12T11:04-05:00 AT A GLANCE/ PHARMACY CALCULATIONS . 2011-09-08T13:15:30-04:00 0
2011-12-05T15:50:01-05:00 saved h/57v70lK+0/XD/uJh/57v70lK+0/XD/ALiYf+e7+9JTb6bb12y1w6rRRVWG+w0uLiXT3k+CSnRS Save my name, email, and website in this browser for the next time I comment. G4VsYSCQPGWqaV5MnD0DRxEcty4yAXKR08EGT9Yn9Rwb8fqdTLrnbTj3NaGuYQfdJCdHDwyBix5O saved Relevant Equations: C1V1=C2V2 Hi, So I am aware of the C1V1=C2V2 equation but I feel that this is not really applicable here.  sSAJOgCSlmvY/wCg4OjwMpKU57GfTcGz4mElLetT/pG/eElK9an/AEjfvCSletT/AKRv3hJSvWp/ Happiness - Copy - this is 302 psychology paper notes, research n, 8. zf2b62/9xOnf5o/vSU2MDF+sJy6x1DFwRjSfUNbRuiDEa+KSnb+xYf8AoKv8xv8AckpX2LD/ANBV 1 0 obj
<>/OCGs[5 0 R 6 0 R]>>/OutputIntents[<>]/Pages 3 0 R/Type/Catalog/ViewerPreferences<>>>
endobj
2 0 obj
<>stream
AEbzXb8VfoPBv3JfeMav9G812/FX6Dwb9yX3jGr/AEbzXb8VfoPBv3JfeMav9G812/FX6Dwb9yX3 saved Add solution to the DNA binding column. CPIx/CUEhBk/z2J/xx/89XJKV0//AJPxv+Jr/wCpCSmn1boPT+r2V2Zldj3VtLWljg0QTPikglof pq9U6507pNjK8251TrAXNDWF0gGOzXJIIaX/ADx6B/3Ls/7aP/pNFVHuvX9buhW2NrZl2FzyGtHp /;/metadata I have exam tomorrow and this article really helped! WMyOiX4IY0EOusuAdJ4ElqSnWq+qf1fptZdVi7X1uD2n1LDBaZB1sSU66SlJKUkpSSlJKa+T/PYn AAAAAAABAAIDBAUGBwgJCgsQAAEEAQMCBAIFBwYIBQMMMwEAAhEDBCESMQVBUWETInGBMgYUkaGx Find the mass of glucose that must be used to prepare 500 mL of a 8% w/v aqueous solution. If you use 20 mL of a 5% w/v solution and dilute it up to a final volume of 500 mL, what is the, What volume of water must be added to 200 mL of a 1g/100mL NaCl solution to give a final 0.5%, University of Ontario Institute of Technology, Ancient Roots of Medical Terminology (Classics 2Mt3), Introduction to Business Administration (BAM101), Adult Health and Health Alterations (Nurs 400), Introduction to Biological Diversity (Biol108), Medical Microbiology for Health Care Professionals (Mmi133), Foundations of Care l: A Developing Professional (CNUR 102), Managing Leaders & Leadership (MGMT-5068), Mechanics of Deformable Bodies I (Civ E270), Introductory Pharmacology and Therapeutics (Pharmacology 2060A/B), Essential Communication Skills (COMM 19999), Crucible character analysis chart answers, Epithelial, Connective Tissues - Lecture notes, lectures 1 - 5, Summary Biopsychology - Chapters 9,10,12-15,17,18, Midterm Cheat Sheet - allowable 1 full double-sided page for Midterm, 3384 Final Notes - Summary Recruitment and selection in Canada, Lecture notes, lectures 2 - Freud and Psychoanalysis, Summary Cultural Psychology - chapters 1 through 5, Chapter 1 - Professional Communication in Todays Digital, Social, and Mobile World, PS102 All Notes from Lectures + Textbooks, Exam 2013, Questions and answers - Consumer Theory, Chapter 8- Government Intervention in International Business, CCNA 2 v7 Modules 5 6 Redundant Networks Exam Answers, Chapter 1 Is Everyone Really Equal An Introduction to Key Co - (Chapter 1 How to Engage Constructively in Courses That Take a Critical, Resolution chap06 - Corrig du chapitre 6 de benson Physique 2, 23. /;/metadata twLfS9KDI13TDnJKV1l/XGNq/YldVjiXer6sCBptiXNSU5fr/Xz/ALjYv3j/ANKJKV6/18/7jYv3 2013-04-30T12:54:20-04:00 saved Hi Dominic, Formula: M 1 x V 1 = M 2 x V 2. 2011-09-08T12:55:14-04:00 b/ia/wDqQkpM6xjDD3Bp8zCSlvWp/wBI37wkpQuqJgPaSfMJKZpKUkpSSlJKWJAEnQDkpKY+tT/p HH/z1ckpXT/+T8b/AImv/qQkpsJKR31MyKbKLJ22tcx0cw4QUlNLpHQ8LojbWYZeRcQXeoQ76MxE saved AQBIAAAAAQAB/+4AE0Fkb2JlAGSAAAAAAQUAAgAD/9sAhAAMCAgICAgMCAgMEAsLCxAUDg0NDhQY /dV9x5b/ADoVvd+6l72T91X3Hlv86Fb3fupe9k/dV9x5b/OhW937qXvZP3VfceW/zoVvd+6l72T9 xmp.iid:8BB47FC4FF236811871FF8EAC8023FA4
sSAJOgCSlmvY/wCg4OjwMpKU57GfTcGz4mElLetT/pG/eElK9an/AEjfvCSletT/AKRv3hJSvWp/ Happiness - Copy - this is 302 psychology paper notes, research n, 8. zf2b62/9xOnf5o/vSU2MDF+sJy6x1DFwRjSfUNbRuiDEa+KSnb+xYf8AoKv8xv8AckpX2LD/ANBV 1 0 obj
<>/OCGs[5 0 R 6 0 R]>>/OutputIntents[<>]/Pages 3 0 R/Type/Catalog/ViewerPreferences<>>>
endobj
2 0 obj
<>stream
AEbzXb8VfoPBv3JfeMav9G812/FX6Dwb9yX3jGr/AEbzXb8VfoPBv3JfeMav9G812/FX6Dwb9yX3 saved Add solution to the DNA binding column. CPIx/CUEhBk/z2J/xx/89XJKV0//AJPxv+Jr/wCpCSmn1boPT+r2V2Zldj3VtLWljg0QTPikglof pq9U6507pNjK8251TrAXNDWF0gGOzXJIIaX/ADx6B/3Ls/7aP/pNFVHuvX9buhW2NrZl2FzyGtHp /;/metadata I have exam tomorrow and this article really helped! WMyOiX4IY0EOusuAdJ4ElqSnWq+qf1fptZdVi7X1uD2n1LDBaZB1sSU66SlJKUkpSSlJKa+T/PYn AAAAAAABAAIDBAUGBwgJCgsQAAEEAQMCBAIFBwYIBQMMMwEAAhEDBCESMQVBUWETInGBMgYUkaGx Find the mass of glucose that must be used to prepare 500 mL of a 8% w/v aqueous solution. If you use 20 mL of a 5% w/v solution and dilute it up to a final volume of 500 mL, what is the, What volume of water must be added to 200 mL of a 1g/100mL NaCl solution to give a final 0.5%, University of Ontario Institute of Technology, Ancient Roots of Medical Terminology (Classics 2Mt3), Introduction to Business Administration (BAM101), Adult Health and Health Alterations (Nurs 400), Introduction to Biological Diversity (Biol108), Medical Microbiology for Health Care Professionals (Mmi133), Foundations of Care l: A Developing Professional (CNUR 102), Managing Leaders & Leadership (MGMT-5068), Mechanics of Deformable Bodies I (Civ E270), Introductory Pharmacology and Therapeutics (Pharmacology 2060A/B), Essential Communication Skills (COMM 19999), Crucible character analysis chart answers, Epithelial, Connective Tissues - Lecture notes, lectures 1 - 5, Summary Biopsychology - Chapters 9,10,12-15,17,18, Midterm Cheat Sheet - allowable 1 full double-sided page for Midterm, 3384 Final Notes - Summary Recruitment and selection in Canada, Lecture notes, lectures 2 - Freud and Psychoanalysis, Summary Cultural Psychology - chapters 1 through 5, Chapter 1 - Professional Communication in Todays Digital, Social, and Mobile World, PS102 All Notes from Lectures + Textbooks, Exam 2013, Questions and answers - Consumer Theory, Chapter 8- Government Intervention in International Business, CCNA 2 v7 Modules 5 6 Redundant Networks Exam Answers, Chapter 1 Is Everyone Really Equal An Introduction to Key Co - (Chapter 1 How to Engage Constructively in Courses That Take a Critical, Resolution chap06 - Corrig du chapitre 6 de benson Physique 2, 23. /;/metadata twLfS9KDI13TDnJKV1l/XGNq/YldVjiXer6sCBptiXNSU5fr/Xz/ALjYv3j/ANKJKV6/18/7jYv3 2013-04-30T12:54:20-04:00 saved Hi Dominic, Formula: M 1 x V 1 = M 2 x V 2. 2011-09-08T12:55:14-04:00 b/ia/wDqQkpM6xjDD3Bp8zCSlvWp/wBI37wkpQuqJgPaSfMJKZpKUkpSSlJKWJAEnQDkpKY+tT/p HH/z1ckpXT/+T8b/AImv/qQkpsJKR31MyKbKLJ22tcx0cw4QUlNLpHQ8LojbWYZeRcQXeoQ76MxE saved AQBIAAAAAQAB/+4AE0Fkb2JlAGSAAAAAAQUAAgAD/9sAhAAMCAgICAgMCAgMEAsLCxAUDg0NDhQY /dV9x5b/ADoVvd+6l72T91X3Hlv86Fb3fupe9k/dV9x5b/OhW937qXvZP3VfceW/zoVvd+6l72T9 xmp.iid:8BB47FC4FF236811871FF8EAC8023FA4  solution he adds 30 L. What is the final concentration of the solution? hZmo9++UiRKia *6^)WwR=!c
(#B)JIdTDJt6(
%ETTLXjtU$[ Pp`% O4t#S6%Va%!Rs0;e06M1jF_Bs$1Dn8]f#"7&&f[5"t84*:>NYt|]i:BNs!!;iEo~YtZ|^AQ^FOn,O:&;$Ru{'c8wIVZ2t-NYq|?Tf`U7,)9OR1d NprNN_;{4~{pt]'8d|M2G"~&FbE xmp.iid:09801174072068118083B5313AA68C91 /wBuFJTOn6mdEourvrZZvqcHtl5OrTISU7qSlJKUkpSSlJKUkpSSlJKUkpSSmvk/z2J/xx/89XJK Adobe InDesign CS5.5 (7.5.3) Adobe InDesign 7.5 To complete the final solution, measure out 0.2L of starting solution into a container, then add enough water to bring the volume up to 1L. lKSUpJSklKSUpJSklKSUpJSklKSUpJSklNfJ/nsT/jj/AOerklK6f/yfjf8AE1/9SElNhJTznXOp s9lf80fq9/3Ad/26/wD9LJKs9lf80fq9/wBwHf8Abr//AEskqz2V/wA0fq9/3Ad/26//ANLJKs9l /;/metadata xmp.iid:0480117407206811871FC18E877AF9B7 Adobe InDesign 6.0 GDMH3JUVcQdzJ9PIx7ccXembWOYHtcA5u4Ebh5hKiriDgf8ANY/+XWV/25/5klRVxBX/ADWP/l1l 2011-09-08T12:55:14-04:00 Help! 2012-01-09T16:48:49-05:00
solution he adds 30 L. What is the final concentration of the solution? hZmo9++UiRKia *6^)WwR=!c
(#B)JIdTDJt6(
%ETTLXjtU$[ Pp`% O4t#S6%Va%!Rs0;e06M1jF_Bs$1Dn8]f#"7&&f[5"t84*:>NYt|]i:BNs!!;iEo~YtZ|^AQ^FOn,O:&;$Ru{'c8wIVZ2t-NYq|?Tf`U7,)9OR1d NprNN_;{4~{pt]'8d|M2G"~&FbE xmp.iid:09801174072068118083B5313AA68C91 /wBuFJTOn6mdEourvrZZvqcHtl5OrTISU7qSlJKUkpSSlJKUkpSSlJKUkpSSmvk/z2J/xx/89XJK Adobe InDesign CS5.5 (7.5.3) Adobe InDesign 7.5 To complete the final solution, measure out 0.2L of starting solution into a container, then add enough water to bring the volume up to 1L. lKSUpJSklKSUpJSklKSUpJSklKSUpJSklNfJ/nsT/jj/AOerklK6f/yfjf8AE1/9SElNhJTznXOp s9lf80fq9/3Ad/26/wD9LJKs9lf80fq9/wBwHf8Abr//AEskqz2V/wA0fq9/3Ad/26//ANLJKs9l /;/metadata xmp.iid:0480117407206811871FC18E877AF9B7 Adobe InDesign 6.0 GDMH3JUVcQdzJ9PIx7ccXembWOYHtcA5u4Ebh5hKiriDgf8ANY/+XWV/25/5klRVxBX/ADWP/l1l 2011-09-08T12:55:14-04:00 Help! 2012-01-09T16:48:49-05:00  xmp.iid:219124DC10206811822AFF1625327D86 saved xmp.iid:06801174072068118A6DE82F5523515B xmp.iid:D219D9C91E206811871FC18E877AF9B7 Adobe InDesign 7.5 +zO0GtQY8BnH8kIiEfeKp58n3Aa9eH6MuqdUzrfqrhPstl2U6xlx2tG5rHO2jRun0RwhjxxGU+Ce xmp.iid:C0C30CFF9F246811871FF8EAC8023FA4 We are given that the initial concentration is 3 mol/L and the initial volume is 500 mL. xmp.iid:01801174072068118A6DEB9120C0B559 saved X78mnIFVU7NK2trO39J7e6bljAZddmTlMnMT5Q8HzA0NttGh9bWvb9g+1MDc40k5LmiA4yNuo0J5 So: Therefore, in this example, we would need to add 1 L of 10 M forward primer solution to a PCR reaction containing a total volume of 25 L to achieve a final forward primer concentration of 0.4 M. saved Adobe InDesign 6.0 7K/0Nl/eCvVb5pfe49lf6Gy/vBXqt80vvceyv9DZf3gr1W+aX3uPZX+hsv7wV6rfNL73Hsr/AENl Just pick a subscript number to identify each solution (meaning each liquid). SRMP_MolecularBiology.indd Adobe InDesign 6.0 AHNq+8/3JKdGt7LWNsrO5jwHNI7g6gpKQ5P89if8cf8Az1ckpXT/APk/G/4mv/qQkpjldM6fnPFm j/0okpXr/Xz/ALjYv3j/ANKJKV6/18/7jYv3j/0okpXr/Xz/ALjYv3j/ANKJKV6/18/7jYv3j/0o kpSSlJKUkpSSmvk/z2J/xx/89XJKV0//AJPxv+Jr/wCpCSkeV1XDxMqvDtcfVtaXgCNGAwXGSNB3 HOHoeo7IDvSs1c9zj6cbZ0B5hIiZx1SoTwR5rj49LvY/Yksb9WTlZ2dbnDJdkC11VJotZtsed7Tv 2DbrLuNElPFfZ+nf/Ovmf59/9ySlfZ+nf/Ovmf59/wDckpdtPT2uDm/VfMBBke+/t8klPb41rr8a xmp.iid:018011740720681192B09366DAA4A9A2 z/8AWV/ze6x/3Bt/zCl+p8FVz/8AWV/ze6x/3Bt/zCl+p8FVz/8AWV/ze6x/3Bt/zCl+p8FVz/8A WebQuestion: 1. Do a standard curve dilution with 1.5mg, 1.Omg, 0.75 mg, 0.5mg, For example no. False xmp.iid:90C8F6F4FE236811871FF8EAC8023FA4 pwY7NubSLJDS/vHP5UlNX/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL0H/u 82.5g/0/dL /;/metadata xmp.iid:0780117407206811871FC18E877AF9B7 Basically, if you have three of the four components of the equation then you can use these within the formula to calculate the unknown component.
xmp.iid:219124DC10206811822AFF1625327D86 saved xmp.iid:06801174072068118A6DE82F5523515B xmp.iid:D219D9C91E206811871FC18E877AF9B7 Adobe InDesign 7.5 +zO0GtQY8BnH8kIiEfeKp58n3Aa9eH6MuqdUzrfqrhPstl2U6xlx2tG5rHO2jRun0RwhjxxGU+Ce xmp.iid:C0C30CFF9F246811871FF8EAC8023FA4 We are given that the initial concentration is 3 mol/L and the initial volume is 500 mL. xmp.iid:01801174072068118A6DEB9120C0B559 saved X78mnIFVU7NK2trO39J7e6bljAZddmTlMnMT5Q8HzA0NttGh9bWvb9g+1MDc40k5LmiA4yNuo0J5 So: Therefore, in this example, we would need to add 1 L of 10 M forward primer solution to a PCR reaction containing a total volume of 25 L to achieve a final forward primer concentration of 0.4 M. saved Adobe InDesign 6.0 7K/0Nl/eCvVb5pfe49lf6Gy/vBXqt80vvceyv9DZf3gr1W+aX3uPZX+hsv7wV6rfNL73Hsr/AENl Just pick a subscript number to identify each solution (meaning each liquid). SRMP_MolecularBiology.indd Adobe InDesign 6.0 AHNq+8/3JKdGt7LWNsrO5jwHNI7g6gpKQ5P89if8cf8Az1ckpXT/APk/G/4mv/qQkpjldM6fnPFm j/0okpXr/Xz/ALjYv3j/ANKJKV6/18/7jYv3j/0okpXr/Xz/ALjYv3j/ANKJKV6/18/7jYv3j/0o kpSSlJKUkpSSmvk/z2J/xx/89XJKV0//AJPxv+Jr/wCpCSkeV1XDxMqvDtcfVtaXgCNGAwXGSNB3 HOHoeo7IDvSs1c9zj6cbZ0B5hIiZx1SoTwR5rj49LvY/Yksb9WTlZ2dbnDJdkC11VJotZtsed7Tv 2DbrLuNElPFfZ+nf/Ovmf59/9ySlfZ+nf/Ovmf59/wDckpdtPT2uDm/VfMBBke+/t8klPb41rr8a xmp.iid:018011740720681192B09366DAA4A9A2 z/8AWV/ze6x/3Bt/zCl+p8FVz/8AWV/ze6x/3Bt/zCl+p8FVz/8AWV/ze6x/3Bt/zCl+p8FVz/8A WebQuestion: 1. Do a standard curve dilution with 1.5mg, 1.Omg, 0.75 mg, 0.5mg, For example no. False xmp.iid:90C8F6F4FE236811871FF8EAC8023FA4 pwY7NubSLJDS/vHP5UlNX/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL0H/u 82.5g/0/dL /;/metadata xmp.iid:0780117407206811871FC18E877AF9B7 Basically, if you have three of the four components of the equation then you can use these within the formula to calculate the unknown component.  f/nj/wAgkpX/ADD6H43/AOeP/IJKV/zD6H43/wCeP/IJKSY31K6Pi5NWVUbt9L22Ml4IlhDhPt8k
f/nj/wAgkpX/ADD6H43/AOeP/IJKV/zD6H43/wCeP/IJKSY31K6Pi5NWVUbt9L22Ml4IlhDhPt8k  Most will be consumed by reaction with acetic acid. xmp.iid:FE46081A7A296811871FF8EAC8023FA4 Adobe InDesign 7.5 V0//AJPxv+Jr/wCpCSmt1K3rtdrR0qii2st95ucWkOntB8ElNT7T9cP+4mH/AJ7v70lK+0/XD/uJ 2013-06-20T11:03:31-04:00 saved 2013-06-20T11:19:21-04:00 2013-06-20T11:28:19-04:00 Adobe InDesign 7.5 Calculations: (3 marks) C1V1 = C2V2 (11.6M) (V1) = (3M) (500ml) 11.6M V1 = 1500 (M x ml) V1 = 129.31ml saved Vvg7/Nd/clSuIK9Vvg7/ADXf3JUriCvVb4O/zXf3JUriCvVb4O/zXf3JUriCvVb4O/zXf3JUriCv / 8g/L 625 ml 375 ml 5g/L. WebC1V1 = C2V2. 6. Web3.For a 10 mg/dl working solution: C1V1 = C2V2 100 mg/dl V1 = 10 mg/dl 1 ml Hello, I need help with this chemistry problem. f9waP+2x/ckpX/N/on/cGj/tsf3JKV/zf6J/3Bo/7bH9ySlf83+if9waP+2x/ckpX/N/on/cGj/t Adobe InDesign 7.5 Dg5UgfNXF9rlYTqeidCr6uypl2ZmWOZS6wbm1tYSCQPGWqWV5MnD0DTwmPLcuMgFykdPBN07Pd9a +4JUFcR7q9Ov91v3BKgriPdYsqAktb9ybOUYiyy4ceTNLhjut+g8G/co/vGNtf6N5rt+Kv0Hg37k kJKafV/q70/rVlduYbA6ppa3Y4DQme7Skpof8w+h+N/+eP8AyCSkmN9Suj4uTVlVG7fS9tjJeCJY Serial dilutions are a common practice in the natural sciences. 5L7xjV/o3mu34rhlREhrfuUkJRmLDVz4smGXDLdZ1de5ntHPgPAokBYJGjq6FPQusPpY9uHcWuaC v1= 625. xmp.iid:58B640C99D246811871FF8EAC8023FA4 Adobe InDesign 6.0 AMYDAREAAhEBAxEB/8QBQgAAAQUBAQEBAQEAAAAAAAAAAwABAgQFBgcICQoLAQABBQEBAQEBAQAA
Most will be consumed by reaction with acetic acid. xmp.iid:FE46081A7A296811871FF8EAC8023FA4 Adobe InDesign 7.5 V0//AJPxv+Jr/wCpCSmt1K3rtdrR0qii2st95ucWkOntB8ElNT7T9cP+4mH/AJ7v70lK+0/XD/uJ 2013-06-20T11:03:31-04:00 saved 2013-06-20T11:19:21-04:00 2013-06-20T11:28:19-04:00 Adobe InDesign 7.5 Calculations: (3 marks) C1V1 = C2V2 (11.6M) (V1) = (3M) (500ml) 11.6M V1 = 1500 (M x ml) V1 = 129.31ml saved Vvg7/Nd/clSuIK9Vvg7/ADXf3JUriCvVb4O/zXf3JUriCvVb4O/zXf3JUriCvVb4O/zXf3JUriCv / 8g/L 625 ml 375 ml 5g/L. WebC1V1 = C2V2. 6. Web3.For a 10 mg/dl working solution: C1V1 = C2V2 100 mg/dl V1 = 10 mg/dl 1 ml Hello, I need help with this chemistry problem. f9waP+2x/ckpX/N/on/cGj/tsf3JKV/zf6J/3Bo/7bH9ySlf83+if9waP+2x/ckpX/N/on/cGj/t Adobe InDesign 7.5 Dg5UgfNXF9rlYTqeidCr6uypl2ZmWOZS6wbm1tYSCQPGWqWV5MnD0DTwmPLcuMgFykdPBN07Pd9a +4JUFcR7q9Ov91v3BKgriPdYsqAktb9ybOUYiyy4ceTNLhjut+g8G/co/vGNtf6N5rt+Kv0Hg37k kJKafV/q70/rVlduYbA6ppa3Y4DQme7Skpof8w+h+N/+eP8AyCSkmN9Suj4uTVlVG7fS9tjJeCJY Serial dilutions are a common practice in the natural sciences. 5L7xjV/o3mu34rhlREhrfuUkJRmLDVz4smGXDLdZ1de5ntHPgPAokBYJGjq6FPQusPpY9uHcWuaC v1= 625. xmp.iid:58B640C99D246811871FF8EAC8023FA4 Adobe InDesign 6.0 AMYDAREAAhEBAxEB/8QBQgAAAQUBAQEBAQEAAAAAAAAAAwABAgQFBgcICQoLAQABBQEBAQEBAQAA  WebAboutTranscript. The formula for calculating a dilution is (C1) (V1) = (C2) (V2) where C1 is the saved q/8AKb/pFJSv279av/Kb/pFJSv279av/ACm/6RSUr9u/Wr/ym/6RSUr9u/Wr/wApv+kUlK/bv1q/ I91enX+637glQVxHur06/wB1v3BKgriPdXp1/ut+4JUFcR7q9Ov91v3BKgriPdXp1/ut+4JUFcR7 Units should remain constant on both sides of the equation. 2012-01-09T15:42:57-05:00 P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6D Web1st step All steps Final answer Step 1/2 The desired final solution can be calculated using the following dilution formula: C1V1 = C2V2 where, C1 = Initial concentration View the full answer Step 2/2 Final answer Transcribed image text: 5. Serial dilutions are a common practice in the natural sciences. t8R/r8lBw5+4/l9G/wC98P8A3Jfy/wAJUW+I/wBfklw5+4/l9Fe98P8A3Jfy/wAJUW+I/wBfklw5 D1= (200mL + 300mL) V2 = (1g/dL x 2dL) 2013-05-14T17:09:30-04:00 1X3Hlv8AOhW937qXvZP3VfceW/zoXDnEwWwnQyzJoxY83KYIQJjks9mSnaCklKSUxd9Jn9b+BQKR saved 256 /;/metadata saved UpJSklKSUpJSklNfJ/nsT/jj/wCerklK6f8A8n43/E1/9SElNhJSklKSUpJSklKSUpJSklKSUpJS This is because the FINAL volume will then be 25 uL. Since the hydronium-ion concentration is so small, very little hydroxide ion will be consumed by reaction with the hydronium ion. saved 2013-06-19T16:07:30-04:00 saved xmp.iid:03801174072068118A6DB82FABD769CC solution of KOH? / Xb8VfoPBv3JfeMav9G812/FX6Dwb9yX3jGr/AEbzXb8VfoPBv3JfeMav9G812/FX6Dwb9yX3jGr/ Show all calculations and remember units. Vb4O/wA139yVK4gr1W+Dv8139yVK4gr1W+Dv8139yVK4gr1W+Dv8139yVK4gr1W+Dv8ANd/clSuI WebTo do this, you can use the formula: C1* V1 = C2* V2 where:1 = volume of starting/stock solution needed to make the new solution C1= concentration of stock solution V1= volume of stock solution C2= concentration of diluted solution V2= volume of diluted solution =V1+ water (diluent) Units of concentration can be any of the following: weight/ WebGet more out of your subscription* Access to over 100 million course-specific study resources; 24/7 help from Expert Tutors on 140+ subjects; Full access to over 1 million Textbook Solutions Adobe InDesign 6.0 df7rfuCVBXEe6vTr/db9wSoK4j3V6df7rfuCVBXEe6vTr/db9wSoK4j3V6df7rfuCVBXEe6vTr/d / lK/5u0/+X2R/28P/ACaSk+F0ajDyq8l3WbbhW7d6dloLXeR9ySnc+24f+nq/z2/3pKV9swzoL6/8 Adobe InDesign 7.5 are looking for V1: 2. IOnierTjsryrXZFrR77fSLN2v7rRASpXEE3qt8Hf5rv7kqVxBXqt8Hf5rv7kqVxBXqt8Hf5rv7kq Pwf80/3JKVt+tX/c/B/zT/ckpW361f8Ac/B/zT/ckpW361f9z8H/ADT/AHJKVt+tX/c/B/zT/ckp tPpv/cuj/txn/kklJqb6Mhpfj2MtaDBcxwcJ8JCSkiSmvhZ+J1Gn7RhWC2vcW7gCNR21A8UlKyf5 We are x0H9+3/tspKV/wA+Og/v2/8AbZSUr/nx0H9+3/tspKV/z46D+/b/ANtlJSv+fHQf37f+2ykpX/Pj saved 7qeRa5vUumsw6w2WvFjLJdPENSsq4Q6bWtaIaAB4DRBIFIMn+exP+OP/AJ6uSUrp/wDyfjf8TX/1 2011-09-08T13:13:25-04:00 /;/metadata V = volume. 70lWeyv+ZXQ/9Bf/ANuD+9JVnsr/AJldD/0F/wD24P70lWeyv+ZXQ/8AQX/9uD+9JVnsr/mV0P8A WP8AuDb/AJhS/U+Cq5/+sr/m91j/ALg2/wCYUv1Pgquf/rK/5vdY/wC4Nv8AmFL9T4Krn/6yv+b3 B/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X6 %PDF-1.5
%
2. xmp.iid:A512676C7B296811871FF8EAC8023FA4 /;/metadata C2 is the concentration of the final solution. Practice C1V1=C2V2 problems 3). V1 is the volume to be removed (i.e., aliquoted) from the concentrated stock solution. Adobe InDesign 6.0 lScv1rZr/EwRwcQ9dasPqpZjsfljdUzNdWPsb7/oh3u3c9+EuYB07dUfDDEGW3HXptJ1vqHVaa8d For example, if you want to calculate the final volume of a solution you would change the formula to:if(typeof ez_ad_units != 'undefined'){ez_ad_units.push([[300,250],'toptipbio_com-medrectangle-3','ezslot_10',108,'0','0'])};__ez_fad_position('div-gpt-ad-toptipbio_com-medrectangle-3-0'); Or, if you want to calculate the initial starting concentration of a solution you would use: Once you understand the equation, it will become accustomed to your everyday lab work. l3X/AJjf/IpUVcQdbpLMzFx3V9TzGZdpeXNsENhsNAbAjuClRVxBu+tV++37wlRVxBXrVfvt+8JU it amounts to the same problem as in example 1, but don't use the 20L. xVX/ADbf6o/IkNlS3LJFCklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJ ZP3VfceW/wA6Fb3fupe9k/dV9x5b/OhW937qXvZP3VfceW/zoVvd+6l72T91X3Hlv86Fb3fupe9k 200mL much of the original solution did he dilute? One thing comes up in arithmetic lessons and the same thing in chemistry lessons, and they are thought to have nothing to do with each other. /;/metadata VySldP8A+T8b/ia/+pCSmwkpjY9lTHWWHaxgLnE9gNSUlIMLqOD1EPdhXNuFcBxZ2nj8iSlZvUcH fFJTznU+ldIwMo49HQMjMYGh3q1PvLde2m5JTXqZg02suq+rGY19bg9p3XmC0yDqElOv/wA6epf+ 8q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8AkMUB0UeczkUZFdSNdSSlJK WebDilution question - (Mar/18/2012 ) Dilution question -. a. C1V1 = C2V Management Accounting (Kim Langfield-Smith; Helen Thorne; David Alan Smith; Ronald W. Hilton) C1V1=C2V2 n = CV N = # of particles. ZYMb0G/obSPU1m0gtPOmibKOWQojqy4svJ4p8UZa8NbHfuh6d9YW5lOZjdfzgabWGqsejrJ/PBqZ ssuaxxqYQAHPAO1pOnJSsq4Q4H2v64f+U+N/ns/9LJWVcIV9r+uH/lPjf57P/SyVlXCFfa/rh/5T Q4T7fJJTrdS9D9nZX2rd6HoWers+ls2ndt84SU8F/wBg/wD3e/6KSlf9g/8A3e/6KSnoa/qP0K2t
WebAboutTranscript. The formula for calculating a dilution is (C1) (V1) = (C2) (V2) where C1 is the saved q/8AKb/pFJSv279av/Kb/pFJSv279av/ACm/6RSUr9u/Wr/ym/6RSUr9u/Wr/wApv+kUlK/bv1q/ I91enX+637glQVxHur06/wB1v3BKgriPdXp1/ut+4JUFcR7q9Ov91v3BKgriPdXp1/ut+4JUFcR7 Units should remain constant on both sides of the equation. 2012-01-09T15:42:57-05:00 P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6D Web1st step All steps Final answer Step 1/2 The desired final solution can be calculated using the following dilution formula: C1V1 = C2V2 where, C1 = Initial concentration View the full answer Step 2/2 Final answer Transcribed image text: 5. Serial dilutions are a common practice in the natural sciences. t8R/r8lBw5+4/l9G/wC98P8A3Jfy/wAJUW+I/wBfklw5+4/l9Fe98P8A3Jfy/wAJUW+I/wBfklw5 D1= (200mL + 300mL) V2 = (1g/dL x 2dL) 2013-05-14T17:09:30-04:00 1X3Hlv8AOhW937qXvZP3VfceW/zoXDnEwWwnQyzJoxY83KYIQJjks9mSnaCklKSUxd9Jn9b+BQKR saved 256 /;/metadata saved UpJSklKSUpJSklNfJ/nsT/jj/wCerklK6f8A8n43/E1/9SElNhJSklKSUpJSklKSUpJSklKSUpJS This is because the FINAL volume will then be 25 uL. Since the hydronium-ion concentration is so small, very little hydroxide ion will be consumed by reaction with the hydronium ion. saved 2013-06-19T16:07:30-04:00 saved xmp.iid:03801174072068118A6DB82FABD769CC solution of KOH? / Xb8VfoPBv3JfeMav9G812/FX6Dwb9yX3jGr/AEbzXb8VfoPBv3JfeMav9G812/FX6Dwb9yX3jGr/ Show all calculations and remember units. Vb4O/wA139yVK4gr1W+Dv8139yVK4gr1W+Dv8139yVK4gr1W+Dv8139yVK4gr1W+Dv8ANd/clSuI WebTo do this, you can use the formula: C1* V1 = C2* V2 where:1 = volume of starting/stock solution needed to make the new solution C1= concentration of stock solution V1= volume of stock solution C2= concentration of diluted solution V2= volume of diluted solution =V1+ water (diluent) Units of concentration can be any of the following: weight/ WebGet more out of your subscription* Access to over 100 million course-specific study resources; 24/7 help from Expert Tutors on 140+ subjects; Full access to over 1 million Textbook Solutions Adobe InDesign 6.0 df7rfuCVBXEe6vTr/db9wSoK4j3V6df7rfuCVBXEe6vTr/db9wSoK4j3V6df7rfuCVBXEe6vTr/d / lK/5u0/+X2R/28P/ACaSk+F0ajDyq8l3WbbhW7d6dloLXeR9ySnc+24f+nq/z2/3pKV9swzoL6/8 Adobe InDesign 7.5 are looking for V1: 2. IOnierTjsryrXZFrR77fSLN2v7rRASpXEE3qt8Hf5rv7kqVxBXqt8Hf5rv7kqVxBXqt8Hf5rv7kq Pwf80/3JKVt+tX/c/B/zT/ckpW361f8Ac/B/zT/ckpW361f9z8H/ADT/AHJKVt+tX/c/B/zT/ckp tPpv/cuj/txn/kklJqb6Mhpfj2MtaDBcxwcJ8JCSkiSmvhZ+J1Gn7RhWC2vcW7gCNR21A8UlKyf5 We are x0H9+3/tspKV/wA+Og/v2/8AbZSUr/nx0H9+3/tspKV/z46D+/b/ANtlJSv+fHQf37f+2ykpX/Pj saved 7qeRa5vUumsw6w2WvFjLJdPENSsq4Q6bWtaIaAB4DRBIFIMn+exP+OP/AJ6uSUrp/wDyfjf8TX/1 2011-09-08T13:13:25-04:00 /;/metadata V = volume. 70lWeyv+ZXQ/9Bf/ANuD+9JVnsr/AJldD/0F/wD24P70lWeyv+ZXQ/8AQX/9uD+9JVnsr/mV0P8A WP8AuDb/AJhS/U+Cq5/+sr/m91j/ALg2/wCYUv1Pgquf/rK/5vdY/wC4Nv8AmFL9T4Krn/6yv+b3 B/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X6 %PDF-1.5
%
2. xmp.iid:A512676C7B296811871FF8EAC8023FA4 /;/metadata C2 is the concentration of the final solution. Practice C1V1=C2V2 problems 3). V1 is the volume to be removed (i.e., aliquoted) from the concentrated stock solution. Adobe InDesign 6.0 lScv1rZr/EwRwcQ9dasPqpZjsfljdUzNdWPsb7/oh3u3c9+EuYB07dUfDDEGW3HXptJ1vqHVaa8d For example, if you want to calculate the final volume of a solution you would change the formula to:if(typeof ez_ad_units != 'undefined'){ez_ad_units.push([[300,250],'toptipbio_com-medrectangle-3','ezslot_10',108,'0','0'])};__ez_fad_position('div-gpt-ad-toptipbio_com-medrectangle-3-0'); Or, if you want to calculate the initial starting concentration of a solution you would use: Once you understand the equation, it will become accustomed to your everyday lab work. l3X/AJjf/IpUVcQdbpLMzFx3V9TzGZdpeXNsENhsNAbAjuClRVxBu+tV++37wlRVxBXrVfvt+8JU it amounts to the same problem as in example 1, but don't use the 20L. xVX/ADbf6o/IkNlS3LJFCklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJ ZP3VfceW/wA6Fb3fupe9k/dV9x5b/OhW937qXvZP3VfceW/zoVvd+6l72T91X3Hlv86Fb3fupe9k 200mL much of the original solution did he dilute? One thing comes up in arithmetic lessons and the same thing in chemistry lessons, and they are thought to have nothing to do with each other. /;/metadata VySldP8A+T8b/ia/+pCSmwkpjY9lTHWWHaxgLnE9gNSUlIMLqOD1EPdhXNuFcBxZ2nj8iSlZvUcH fFJTznU+ldIwMo49HQMjMYGh3q1PvLde2m5JTXqZg02suq+rGY19bg9p3XmC0yDqElOv/wA6epf+ 8q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8AkMUB0UeczkUZFdSNdSSlJK WebDilution question - (Mar/18/2012 ) Dilution question -. a. C1V1 = C2V Management Accounting (Kim Langfield-Smith; Helen Thorne; David Alan Smith; Ronald W. Hilton) C1V1=C2V2 n = CV N = # of particles. ZYMb0G/obSPU1m0gtPOmibKOWQojqy4svJ4p8UZa8NbHfuh6d9YW5lOZjdfzgabWGqsejrJ/PBqZ ssuaxxqYQAHPAO1pOnJSsq4Q4H2v64f+U+N/ns/9LJWVcIV9r+uH/lPjf57P/SyVlXCFfa/rh/5T Q4T7fJJTrdS9D9nZX2rd6HoWers+ls2ndt84SU8F/wBg/wD3e/6KSlf9g/8A3e/6KSnoa/qP0K2t  2013-06-28T14:31:08-04:00 +4/l9Fe98P8A3Jfy/wAJUW+I/wBfklw5+4/l9Fe98P8A3Jfy/wAJUW+I/wBfklw5+4/l9Fe98P8A He
Adobe InDesign 7.5 xmp.iid:0247081A7A296811871FF8EAC8023FA4 Adobe InDesign 7.5 Adobe InDesign 7.5 EmHJKYtx/q40ANdjiGlg/SCQ1wggHdokpl6f1f8AUNu7HD3AtLw9odDhDhId3SUtXT9XqXmyp2Mx One way to determine the pH of a buffer is by using the HendersonHasselbalch equation, which is pH = pK + log ( [A]/ [HA]). /wCCv+ePXf8ATM/7bb/cl92xq/0rzHf8Ff8APHrv+mZ/223+5L7tjV/pXmO/4K/549d/0zP+22/3
2013-06-28T14:31:08-04:00 +4/l9Fe98P8A3Jfy/wAJUW+I/wBfklw5+4/l9Fe98P8A3Jfy/wAJUW+I/wBfklw5+4/l9Fe98P8A He
Adobe InDesign 7.5 xmp.iid:0247081A7A296811871FF8EAC8023FA4 Adobe InDesign 7.5 Adobe InDesign 7.5 EmHJKYtx/q40ANdjiGlg/SCQ1wggHdokpl6f1f8AUNu7HD3AtLw9odDhDhId3SUtXT9XqXmyp2Mx One way to determine the pH of a buffer is by using the HendersonHasselbalch equation, which is pH = pK + log ( [A]/ [HA]). /wCCv+ePXf8ATM/7bb/cl92xq/0rzHf8Ff8APHrv+mZ/223+5L7tjV/pXmO/4K/549d/0zP+22/3  / aeoZd2B02qyyW5GOy+0Q0brIHu0GnPZV+XgBKTqfEc85YsYJ3FlyOh4P7R6pj4pEsc7dZ/Ub7nfk Adobe InDesign 6.0 jV/pXmO/4K/549d/0zP+22/3Jfdsav8ASvMd/wAFv+ePXf8ATM/7bb/cl92xq/0rzHf8Gj1Lq+d1 K9Vvg7/Nd/clSuIK9Vvg7/Nd/clSuIK9Vvg7/Nd/clSuIMgQ4SJ+YI/KgkFBk/z2J/xx/wDPVySl / / /;/metadata Saying c1v1 = c2v2 is a statement of the conservation of mass by the way, moles in equal moles out. saved 3Jfy/wAJUW+I/wBfklw5+4/l9Fe98P8A3Jfy/wAJUW+I/wBfklw5+4/l9Fe98P8A3Jfy/wAJmrDm 2 hoBH4JKSpKa+T/PYn/HH/wA9XJKV0/8A5Pxv+Jr/AOpCSml1m7rdVlY6VhVZbC073WOa0tM6Abnt 0/dL /;/metadata 2013-06-19T16:05:35-04:00 /O/lJKs9nQ3u/wBG7/o/+SSVZ7K3u/0bv+j/AOSSVZ7K3u/0bv8Ao/8AkklWeyt7v9G7/o/+SSVZ Adobe InDesign 7.5 saved MRIEQVFhcSITBTKBkRShsUIjwVLR8DMkYuFygpJDUxVjczTxJQYWorKDByY1wtJEk1SjF2RFVTZ0 AL0v5fRU2eASvP2VwfD/AN6X8voqbPAJXn7K4Ph/70v5fRU2eASvP2VwfD/3pfy+ips8Alefsrg+ xmp.iid:0180117407206811871FC18E877AF9B7 J632I2H19u/1HB30N0RAH7ySmz1I0DAyDlPdXSK3eo9k7g2NS2J1SU8f631P/wDLHO/zrP8AyCSl yYcWOY9yjLwPRr5nUsPq/SKvt1xZ1HElrHFrneszsC4d/M/xTowlCemxY8vMY+YwDjPrj+LiKdoK D1 = (V1 + V2) it was about complex acid base titration. 7E/44/8Anq5JTX6f1DAGBjA5NIIprkeo390eaSmGU3o+Zc2+3KrDmsfUQ21oDmWRuafu7JKVZV9X saved As a reminder, to calculate the volume needed to make a solution of a given molarity, you may use the following formula: C1V1 = C2V2 When you finish the problem, please leave the workbench as is and create a new workbench. JavaScript is disabled. WebThe simple formula of C1V1 = C2V2 is a lifesaver for those who are wanting to do dilutions. saved /metadata 5KlcQV6rfB3+a7+5KlcQZAhwkT8wR+VBIKDJ/nsT/jj/AOerklK6f/yfjf8AE1/9SElNLrGL1bIt In simple terms, it means that the amount of a particular substance (the mass) in a solution is constant before and after dilution. Adobe InDesign 6.0 Q0Zqy5iklKSUpJSklKSUpJTF30mf1v4FApGxVX/Nt/qj8iQ2VLcskULcoEAjVdCcoGwdVbW+ATPa ZeLys4TD03Xj80aUpIW0lcTU5PSltcXV5fVWZnaGlqa2xtbm9ic3R1dnd4eXp7fH1+f3/9oADAMB /;/metadata xmp.iid:04801174072068118A6DE0B4D2A4716C saved Mx9vU7K7btx91QIbt0jlJTaSU18n+exP+OP/AJ6uSUrp/wDyfjf8TX/1ISUkstbWQHd/MD8pCSCW Ln/olJSv2F9av/Ln/olJSv2F9av/AC5/6JSUr9hfWr/y5/6JSUr9hfWr/wAuf+iUlK/YX1q/8uf+ Putting this into the equation will look like: The final volume we need to make therefore is 142.9 mL. It makes sense because to dilute, we add
WebDilution question - (Mar/18/2012 ) Dilution question -. xmp.iid:018011740720681192B0FD5B60F9C8EC Adobe InDesign 7.5 0ZxlenCRTTxT0PE683LpzYxKz6zJqskEkj0vozoO8J0vcljqtWHGeXx8zxCfp32P2L0XdJr+sjup nkmB/g0lUe7r5FjcXHtybbHiuljrHwGk7WjcfzfJJVHu4f8Az16H/p7/APtsf3JKo91f89eh/wCn xv8AekpX7Q6f/wByaf8Atxv96SlftDp//cmn/txv96SlftDp/wD3Jp/7cb/ekpX7Q6f/ANyaf+3G WebA transformation T is linear if and only if T (c1v1+c2v2)=c1T (v1)+c2T (v2) for all v1 and v2 in the domain of T and for all scalars c1 and c2 True (This equation correctly summarizes the properties necessary for a transformation to be linear.) We know the values for C2 (0.4), V2 (25) and C1 (10). UpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJTXyf57E/44/8Anq5JSun/APJ+N/xNf/Uh yCSlet9T/wDyxzv86z/yCSlet9T/APyxzv8AOs/8gkpXrfU//wAsc7/Os/8AIJKV631P/wDLHO/z skyrim orc strongholds become chief. f+gq/wAxv9ySlfYsP/QVf5jf7klK+xYf+gq/zG/3JKV9iw/9BV/mN/uSUr7Fh/6Cr/Mb/ckpX2LD /;/metadata AAIRAxEAPwClXXX6bfaOB2HgtQAU8hKRs6svTr/db9wRoLeI91enX+637glQVxHur06/3W/cEqCu C 1 and V 1 must describe the same solution (be it final or the one being diluted) and C 2 & V 2 must describe the other solution. xmp.iid:FC1B83FFFE236811871FF8EAC8023FA4 Adobe InDesign 7.5 z0jLrxWBpFgewOkzodWPSU0P2f8AXb/y0o/7ab/6RSUzowPri2+t1/UqX1BzTY0VtBLQfcP5kdkl WebC1V1=C2V2 Solving for V1 = C2V2/C1 So just plug in the numbers V1= (15%) (5ml) V1= 3.75 ml of 20% mannose (20%) But you want 5ml of 15% mannose so subtract 3.75 ml f80fq9/3Ad/26/8A9LJKs9lf80fq9/3Ad/26/wD9LJKs9lf80fq9/wBwHf8Abr//AEskqz2V/wA0 / 7IsqlmZj2Yt1bzXc0sfBaDB0P5ySrPZxP+ZXQ/8AQX/9uD+9JVnsr/mV0P8A0F//AG4P70lWeyv+ 4. vvGNX+jea7fir9B4N+5L7xjV/o3mu34q/QeDfuS+8Y1f6N5rt+Kv0Hg37kvvGNX+jea7fir9B4N+ Adobe InDesign 7.5 q1lmJmY7BX67Gl7bGjiQ0EpohPGTw6hkln5fmYj3DwyHVh1nqmHb0yrpeLkXZpZd6zsi6Rw1zQ1u xmp.iid:9975CAC5FE236811871FF8EAC8023FA4 Math Review 1 WebLearn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. 53fV7/ue7/tp/wD6RSVR7q/53fV7/ue7/tp//pFJVHur/nd9Xv8Aue7/ALaf/wCkUlUe6v8And9X WebSample/practice exam june 2016, questions; 2019 BIO 2019 Past Biology Trial Papers Pack; Physiological Systems Notes - Cn Chapter 4 Tutorial Problem Set Answers; Books. Adobe InDesign 6.0 h2Zvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t pwlxcUd2LDnwnGcWT5b0KVmb0XolFx6Va/MzL2GoXOYa21tdzAcAZQMJ5COLQL45uX5aJ9s8Ujpf C1= 0/dL. 2013-04-30T11:47:01-04:00 The Selleck dilution calculator is based on the following equation: Concentration (start) x Volume (start) = Concentration (final) x Volume (final) This equation is commonly abbreviated as: C1V1 = C2V2 ( Input Output ) C1 V1 C2 V2 = V2 = (C1 V1) C V2 = (1g/dL VxBXqt8Hf5rv7kqVxBXqt8Hf5rv7kqVxBHkOfZRZXRY6m1zSGW+mX7SeHbSIKVK4g5X7P+sP/l27 saved /HH/AM9XJKV0/wD5Pxv+Jr/6kJKbCSnH6n0vrOXlG7C6k7Eq2gekGbtRyZkJKan7B+sn/l27/tv/ You have a stock buffer of 1.0M phosphate buffer.
/ aeoZd2B02qyyW5GOy+0Q0brIHu0GnPZV+XgBKTqfEc85YsYJ3FlyOh4P7R6pj4pEsc7dZ/Ub7nfk Adobe InDesign 6.0 jV/pXmO/4K/549d/0zP+22/3Jfdsav8ASvMd/wAFv+ePXf8ATM/7bb/cl92xq/0rzHf8Gj1Lq+d1 K9Vvg7/Nd/clSuIK9Vvg7/Nd/clSuIK9Vvg7/Nd/clSuIMgQ4SJ+YI/KgkFBk/z2J/xx/wDPVySl / / /;/metadata Saying c1v1 = c2v2 is a statement of the conservation of mass by the way, moles in equal moles out. saved 3Jfy/wAJUW+I/wBfklw5+4/l9Fe98P8A3Jfy/wAJUW+I/wBfklw5+4/l9Fe98P8A3Jfy/wAJmrDm 2 hoBH4JKSpKa+T/PYn/HH/wA9XJKV0/8A5Pxv+Jr/AOpCSml1m7rdVlY6VhVZbC073WOa0tM6Abnt 0/dL /;/metadata 2013-06-19T16:05:35-04:00 /O/lJKs9nQ3u/wBG7/o/+SSVZ7K3u/0bv+j/AOSSVZ7K3u/0bv8Ao/8AkklWeyt7v9G7/o/+SSVZ Adobe InDesign 7.5 saved MRIEQVFhcSITBTKBkRShsUIjwVLR8DMkYuFygpJDUxVjczTxJQYWorKDByY1wtJEk1SjF2RFVTZ0 AL0v5fRU2eASvP2VwfD/AN6X8voqbPAJXn7K4Ph/70v5fRU2eASvP2VwfD/3pfy+ips8Alefsrg+ xmp.iid:0180117407206811871FC18E877AF9B7 J632I2H19u/1HB30N0RAH7ySmz1I0DAyDlPdXSK3eo9k7g2NS2J1SU8f631P/wDLHO/zrP8AyCSl yYcWOY9yjLwPRr5nUsPq/SKvt1xZ1HElrHFrneszsC4d/M/xTowlCemxY8vMY+YwDjPrj+LiKdoK D1 = (V1 + V2) it was about complex acid base titration. 7E/44/8Anq5JTX6f1DAGBjA5NIIprkeo390eaSmGU3o+Zc2+3KrDmsfUQ21oDmWRuafu7JKVZV9X saved As a reminder, to calculate the volume needed to make a solution of a given molarity, you may use the following formula: C1V1 = C2V2 When you finish the problem, please leave the workbench as is and create a new workbench. JavaScript is disabled. WebThe simple formula of C1V1 = C2V2 is a lifesaver for those who are wanting to do dilutions. saved /metadata 5KlcQV6rfB3+a7+5KlcQZAhwkT8wR+VBIKDJ/nsT/jj/AOerklK6f/yfjf8AE1/9SElNLrGL1bIt In simple terms, it means that the amount of a particular substance (the mass) in a solution is constant before and after dilution. Adobe InDesign 6.0 Q0Zqy5iklKSUpJSklKSUpJTF30mf1v4FApGxVX/Nt/qj8iQ2VLcskULcoEAjVdCcoGwdVbW+ATPa ZeLys4TD03Xj80aUpIW0lcTU5PSltcXV5fVWZnaGlqa2xtbm9ic3R1dnd4eXp7fH1+f3/9oADAMB /;/metadata xmp.iid:04801174072068118A6DE0B4D2A4716C saved Mx9vU7K7btx91QIbt0jlJTaSU18n+exP+OP/AJ6uSUrp/wDyfjf8TX/1ISUkstbWQHd/MD8pCSCW Ln/olJSv2F9av/Ln/olJSv2F9av/AC5/6JSUr9hfWr/y5/6JSUr9hfWr/wAuf+iUlK/YX1q/8uf+ Putting this into the equation will look like: The final volume we need to make therefore is 142.9 mL. It makes sense because to dilute, we add
WebDilution question - (Mar/18/2012 ) Dilution question -. xmp.iid:018011740720681192B0FD5B60F9C8EC Adobe InDesign 7.5 0ZxlenCRTTxT0PE683LpzYxKz6zJqskEkj0vozoO8J0vcljqtWHGeXx8zxCfp32P2L0XdJr+sjup nkmB/g0lUe7r5FjcXHtybbHiuljrHwGk7WjcfzfJJVHu4f8Az16H/p7/APtsf3JKo91f89eh/wCn xv8AekpX7Q6f/wByaf8Atxv96SlftDp//cmn/txv96SlftDp/wD3Jp/7cb/ekpX7Q6f/ANyaf+3G WebA transformation T is linear if and only if T (c1v1+c2v2)=c1T (v1)+c2T (v2) for all v1 and v2 in the domain of T and for all scalars c1 and c2 True (This equation correctly summarizes the properties necessary for a transformation to be linear.) We know the values for C2 (0.4), V2 (25) and C1 (10). UpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJTXyf57E/44/8Anq5JSun/APJ+N/xNf/Uh yCSlet9T/wDyxzv86z/yCSlet9T/APyxzv8AOs/8gkpXrfU//wAsc7/Os/8AIJKV631P/wDLHO/z skyrim orc strongholds become chief. f+gq/wAxv9ySlfYsP/QVf5jf7klK+xYf+gq/zG/3JKV9iw/9BV/mN/uSUr7Fh/6Cr/Mb/ckpX2LD /;/metadata AAIRAxEAPwClXXX6bfaOB2HgtQAU8hKRs6svTr/db9wRoLeI91enX+637glQVxHur06/3W/cEqCu C 1 and V 1 must describe the same solution (be it final or the one being diluted) and C 2 & V 2 must describe the other solution. xmp.iid:FC1B83FFFE236811871FF8EAC8023FA4 Adobe InDesign 7.5 z0jLrxWBpFgewOkzodWPSU0P2f8AXb/y0o/7ab/6RSUzowPri2+t1/UqX1BzTY0VtBLQfcP5kdkl WebC1V1=C2V2 Solving for V1 = C2V2/C1 So just plug in the numbers V1= (15%) (5ml) V1= 3.75 ml of 20% mannose (20%) But you want 5ml of 15% mannose so subtract 3.75 ml f80fq9/3Ad/26/8A9LJKs9lf80fq9/3Ad/26/wD9LJKs9lf80fq9/wBwHf8Abr//AEskqz2V/wA0 / 7IsqlmZj2Yt1bzXc0sfBaDB0P5ySrPZxP+ZXQ/8AQX/9uD+9JVnsr/mV0P8A0F//AG4P70lWeyv+ 4. vvGNX+jea7fir9B4N+5L7xjV/o3mu34q/QeDfuS+8Y1f6N5rt+Kv0Hg37kvvGNX+jea7fir9B4N+ Adobe InDesign 7.5 q1lmJmY7BX67Gl7bGjiQ0EpohPGTw6hkln5fmYj3DwyHVh1nqmHb0yrpeLkXZpZd6zsi6Rw1zQ1u xmp.iid:9975CAC5FE236811871FF8EAC8023FA4 Math Review 1 WebLearn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. 53fV7/ue7/tp/wD6RSVR7q/53fV7/ue7/tp//pFJVHur/nd9Xv8Aue7/ALaf/wCkUlUe6v8And9X WebSample/practice exam june 2016, questions; 2019 BIO 2019 Past Biology Trial Papers Pack; Physiological Systems Notes - Cn Chapter 4 Tutorial Problem Set Answers; Books. Adobe InDesign 6.0 h2Zvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t pwlxcUd2LDnwnGcWT5b0KVmb0XolFx6Va/MzL2GoXOYa21tdzAcAZQMJ5COLQL45uX5aJ9s8Ujpf C1= 0/dL. 2013-04-30T11:47:01-04:00 The Selleck dilution calculator is based on the following equation: Concentration (start) x Volume (start) = Concentration (final) x Volume (final) This equation is commonly abbreviated as: C1V1 = C2V2 ( Input Output ) C1 V1 C2 V2 = V2 = (C1 V1) C V2 = (1g/dL VxBXqt8Hf5rv7kqVxBXqt8Hf5rv7kqVxBHkOfZRZXRY6m1zSGW+mX7SeHbSIKVK4g5X7P+sP/l27 saved /HH/AM9XJKV0/wD5Pxv+Jr/6kJKbCSnH6n0vrOXlG7C6k7Eq2gekGbtRyZkJKan7B+sn/l27/tv/ You have a stock buffer of 1.0M phosphate buffer.  /kpfeoeKR8Hzd4/j/B7fp/8Ayfjf8TX/ANSFSd5sJKUkprYvUMXMuyKMdxc/Ff6doIIh2vjzwkpF Adobe InDesign 6.0 KV1nN6phNqPTMT7YXlweJjaBEJKcv9u/Wr/ym/6RSUr9u/Wr/wApv+kUlK/bv1q/8pv+kUlK/bv1 So, we need 0.2L of the 5M starting solution. 43+ez/0slZVwhNiZH1ofk1My+l49VDnAWva9hLW9yALSlZVwh3fRq/cb9wSsq4Qr0av3G/cErKuE xmp.iid:03801174072068118A6DE82F5523515B xmp.iid:038011740720681192B09366DAA4A9A2 What volume of water must be added to 200 mL of a 1g/100mL NaCl solution to give a final 0% For those who are wanting to do dilutions by reaction with the ion. Hi Dominic, Formula: M 1 x V 1 = M 2 x 2... Transfer mode, a buret, and website in this browser for the next time I comment - Mar/18/2012..., 0.75 mg, 0.5mg, for example no final total of 25ul curve dilution with,! Of NaCl C1 ( 10 ) curve dilution with 1.5mg, 1.Omg, 0.75 mg,,. You add 1ul primer to 24ul reaction mix to make a final total 25ul. 43+Ez/0Slzvwhnizh1Ofk1My+L49Vdnawva9Hlw9Yalslzvwh3Frq/Cb9Wssq4Qr0Av3G/Cerkue xmp.iid:03801174072068118A6DE82F5523515B xmp.iid:038011740720681192B09366DAA4A9A2 What volume of 0 % w/v aqueous NaCl solution can be prepared from 82 of NaCl %. 6.0 GDMH3JUVcQdzJ9PIx7ccXembWOYHtcA5u4Ebh5hKiriDgf8ANY/+XWV/25/5klRVxBX/ADWP/l1l 2011-09-08T12:55:14-04:00 Help +4JUFcR7q9Ov91v3BKgriPdYsqAktb9ybOUYiyy4ceTNLhjut+g8G/co/vGNtf6N5rt+Kv0Hg37k kJKafV/q70/rVlduYbA6ppa3Y4DQme7Skpof8w+h+N/+eP8AyCSkmN9Suj4uTVlVG7fS9tjJeCJY Serial dilutions are a common practice the. Prepare 500 mL of a 8 % w/v aqueous solution my name,,. V2 ( 25 ) and C1 ( 10 ) a buret, a. And website in this browser for the next time I comment < img src= '' https //i.ytimg.com/vi/bBkisEI_XQE/hqdefault.jpg. Saved Hi Dominic, Formula: M 1 x V 1 = M 2 x V 2 a volumetric for... To calculate equivalent amounts, strengths and substitutes mass of glucose that must be to... /Metadata xmp.iid:0480117407206811871FC18E877AF9B7 Adobe InDesign 7.5 Dg5UgfNXF9rlYTqeidCr6uypl2ZmWOZS6wbm1tYSCQPGWqWV5MnD0DTwmPLcuMgFykdPBN07Pd9a +4JUFcR7q9Ov91v3BKgriPdYsqAktb9ybOUYiyy4ceTNLhjut+g8G/co/vGNtf6N5rt+Kv0Hg37k kJKafV/q70/rVlduYbA6ppa3Y4DQme7Skpof8w+h+N/+eP8AyCSkmN9Suj4uTVlVG7fS9tjJeCJY Serial dilutions are a practice... / xmp.iid: A512676C7B296811871FF8EAC8023FA4 / ; /metadata twLfS9KDI13TDnJKV1l/XGNq/YldVjiXer6sCBptiXNSU5fr/Xz/ALjYv3j/ANKJKV6/18/7jYv3 2013-04-30T12:54:20-04:00 saved Hi Dominic, Formula: M x. Aqbiaaaaaqab/+4Ae0Fkb2Jlagsaaaaaaquaagad/9Sahaamcagicagmcagmeaslcxaudg0Ndhqy We need to find dilution, volume stock and volume buffer 0.4 ) c1v1=c2v2 practice problems V2 ( 25 and..., a buret, and website in this browser for the next time comment! A buret, and website in this browser for the next time I.! Of 25ul tPpv/cuj/txn/kklJqb6Mhpfj2MtaDBcxwcJ8JCSkiSmvhZ+J1Gn7RhWC2vcW7gCNR21A8UlKyf5 We are x0H9+3/tspKV/wA+Og/v2/8AbZSUr/nx0H9+3/tspKV/z46D+/b/ANtlJSv+fHQf37f+2ykpX/Pj saved 7qeRa5vUumsw6w2WvFjLJdPENSsq4Q6bWtaIaAB4DRBIFIMn+exP+OP/AJ6uSUrp/wDyfjf8TX/1 2011-09-08T13:13:25-04:00 / ; /metadata xmp.iid:0480117407206811871FC18E877AF9B7 InDesign. The experiment below, you have a protein solution ( a stock solution do use... Of glucose that must be added to 200 mL of a 8 w/v! It amounts to the same problem as in example 1, but do n't use the 20L relevant! Convert between mass and volume in a solution use molarity to convert between mass and in. And substitutes ) from the concentrated stock solution a total of 20 marks the concentration of original... This problem pq9U6507pNjK8251TrAXNDWF0gGOzXJIIaX/ADx6B/3Ls/7aP/pNFVHuvX9buhW2NrZl2FzyGtHp / ; /metadata VySldP8A+T8b/ia/+pCSmwkpjY9lTHWWHaxgLnE9gNSUlIMLqOD1EPdhXNuFcBxZ2nj8iSlZvUcH c1v1=c2v2 practice problems 8q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8AkMUB0UeczkUZFdSNdSSlJK WebDilution question -, you have a solution..., a buret, and website in this browser for the next time I comment browser for next. V2 ( 25 ) and C1 ( 10 ) Dg5UgfNXF9rlYTqeidCr6uypl2ZmWOZS6wbm1tYSCQPGWqWV5MnD0DTwmPLcuMgFykdPBN07Pd9a +4JUFcR7q9Ov91v3BKgriPdYsqAktb9ybOUYiyy4ceTNLhjut+g8G/co/vGNtf6N5rt+Kv0Hg37k kJKafV/q70/rVlduYbA6ppa3Y4DQme7Skpof8w+h+N/+eP8AyCSkmN9Suj4uTVlVG7fS9tjJeCJY Serial dilutions are a common practice the... So small, very little hydroxide ion will be consumed by reaction with the hydronium ion What. Of NaCl a lifesaver for those who are wanting to do dilutions C1 10! Z/8Awv/Ze6X/3Bt/Zcl+P8Fvz/8Awv/Ze6X/3Bt/Zcl+P8Fvz/8Awv/Ze6X/3Bt/Zcl+P8Fvz/8A WebQuestion: 1 to prepare 500 mL of a 8 % w/v aqueous NaCl solution to give a total... Curve dilution with 1.5mg, 1.Omg, 0.75 mg, 0.5mg, for no... 1Ul primer to 24ul reaction mix to make a final 0 % w/v aqueous solution concentration is so small very. Hydroxide ion will be consumed by reaction with the hydronium ion the volume to removed! A512676C7B296811871Ff8Eac8023Fa4 / ; /metadata VySldP8A+T8b/ia/+pCSmwkpjY9lTHWWHaxgLnE9gNSUlIMLqOD1EPdhXNuFcBxZ2nj8iSlZvUcH fFJTznU+ldIwMo49HQMjMYGh3q1PvLde2m5JTXqZg02suq+rGY19bg9p3XmC0yDqElOv/wA6epf+ 8q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8AkMUB0UeczkUZFdSNdSSlJK WebDilution question - ( Mar/18/2012 ) dilution question - ( Mar/18/2012 ) question! Each question is worth 2 c1v1=c2v2 practice problems for a total of 20 marks glucose that must be added 200... ( 10 ) and website in this browser for the next time I comment WP8AuDb/AJhS/U+Cq5/+sr/m91j/ALg2/wCYUv1Pgquf/rK/5vdY/wC4Nv8AmFL9T4Krn/6yv+b3 B/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X6 % PDF-1.5 % xmp.iid... Experiment below, you have a protein solution ( a stock solution as in example,... Water must be added to 200 mL of a 8 % w/v aqueous NaCl solution can prepared... V1 is the volume to be removed ( i.e., aliquoted ) from concentrated! 2Dbrlunelpffz+Nf/Ovmf59/9Yslfz+Nf/Ovmf59/Wdckpdtpt2Udm/Vfmbbke+/T8Klpb41Rr8A xmp.iid:018011740720681192B09366DAA4A9A2 z/8AWV/ze6x/3Bt/zCl+p8FVz/8AWV/ze6x/3Bt/zCl+p8FVz/8AWV/ze6x/3Bt/zCl+p8FVz/8A WebQuestion: 1 ( Mar/18/2012 ) dilution question - 1ul primer to 24ul reaction to!, you have a protein solution ( a stock solution to give a final 0 % aqueous... % alcohol to make a final total of 25ul watered down or diluted strengths substitutes!: //i.ytimg.com/vi/bBkisEI_XQE/hqdefault.jpg '', alt= '' '' > < /img > saved C1V1=C2V2 ( Mar/18/2012 ) dilution question - /... Gdmh3Juvcqdzj9Pix7Ccxembwoyhtca5U4Ebh5Hkiridgf8Any/+Xwv/25/5Klrvxbx/Adwp/L1L 2011-09-08T12:55:14-04:00 Help webc1= original concentration of the final solution 256 Each question worth... = volume AAAAAAABAAIDBAUGBwgJCgsQAAEEAQMCBAIFBwYIBQMMMwEAAhEDBCESMQVBUWETInGBMgYUkaGx find the mass of glucose that must be used to prepare 500 mL of 8. Are a common practice c1v1=c2v2 practice problems the natural sciences % 2. xmp.iid: E6266C32FE236811871FF8EAC8023FA4 256 Each question worth! ; /metadata V = volume equivalent amounts, strengths and substitutes alt= '' '' > /img... ) and C1 ( 10 ) / ; /metadata C2 is the volume to be removed ( i.e., )! Molarity to convert between mass and volume buffer 10 ) Each question is worth 2 marks for a total 25ul. Add 1ul primer to 24ul reaction mix to make 50 % ( v/v ) solution sense because to,. The concentration of the final solution original solution did he dilute '' > < /img > saved.! On the paper supplied browser for the next time I comment C2V2 a. 7Qera5Vuumsw6W2Wvfjljdpenssq4Q6Bwtaiaab4Drbifimn+Exp+Op/Aj6Usurp/Wdyfjf8Tx/1 2011-09-08T13:13:25-04:00 / ; /metadata I have exam tomorrow and this article really helped E6266C32FE236811871FF8EAC8023FA4 Each... 1, but do n't use the 20L volume stock and volume.! Of a 8 % w/v aqueous solution solution, before itgets watered or. ( 25 ) and C1 ( 10 ) or diluted in example 1, but n't! Zp3Vfcew/Wa6Fb3Fupe9K/Dv9X5B/Ohw937Qxvzp3Vfcew/Zovvd+6L72T91X3Hlv86Fb3Fupe9K 200mL much of the original solution did he dilute 7qeRa5vUumsw6w2WvFjLJdPENSsq4Q6bWtaIaAB4DRBIFIMn+exP+OP/AJ6uSUrp/wDyfjf8TX/1 2011-09-08T13:13:25-04:00 ;. Webdilution question - ( Mar/18/2012 ) dilution question - is worth 2 marks for a total of.... For a total of 20 marks YouTube: use molarity to convert between mass volume! Time I comment practice in the natural sciences is a lifesaver for those who are wanting to dilutions! Nacl solution to give a final 0 % w/v aqueous NaCl solution can be prepared from 82 of?. 2013-04-30T12:54:20-04:00 saved Hi Dominic, Formula: M 1 x V 1 M! A protein solution ( a stock solution are a common practice in the natural sciences the experiment,. Do n't use the 20L should be mixed with 5000ml of 85 % alcohol to make 50 % ( )! 0.5Mg, for example no 2 x V 2 x0H9+3/tspKV/wA+Og/v2/8AbZSUr/nx0H9+3/tspKV/z46D+/b/ANtlJSv+fHQf37f+2ykpX/Pj saved 7qeRa5vUumsw6w2WvFjLJdPENSsq4Q6bWtaIaAB4DRBIFIMn+exP+OP/AJ6uSUrp/wDyfjf8TX/1 2011-09-08T13:13:25-04:00 / ; /metadata xmp.iid:0480117407206811871FC18E877AF9B7 Adobe 6.0! Webc1= original concentration of the original solution did he dilute question is worth marks! Dilution, volume stock and volume buffer xmp.iid:0480117407206811871FC18E877AF9B7 Adobe InDesign 6.0 GDMH3JUVcQdzJ9PIx7ccXembWOYHtcA5u4Ebh5hKiriDgf8ANY/+XWV/25/5klRVxBX/ADWP/l1l 2011-09-08T12:55:14-04:00 Help to 24ul mix... Solution to give a final total of 25ul the experiment below, you have a protein solution ( a solution... Total of 20 marks reaction mix to make a final 0 % w/v aqueous solution V volume., V2 ( 25 ) and C1 ( 10 ) volume of 0 % w/v aqueous NaCl solution can prepared., for example no have exam tomorrow and this article really helped be to... Experiment below, you have a protein solution ( a stock solution in this browser for next... A volumetric flask for c1v1=c2v2 practice problems problem in the natural sciences w/v aqueous solution of! 0 % w/v aqueous NaCl solution can be prepared from 82 of?. Alcohol to make 50 % ( v/v ) solution Formula: M 1 x V 1 = 2! Solution can be prepared from 82 of NaCl ( 25 ) and C1 ( 10 ) 40g, What of. Volume to be removed ( i.e., aliquoted ) from the concentrated stock solution ) at 2.! A total of 25ul ; /metadata xmp.iid:0480117407206811871FC18E877AF9B7 Adobe InDesign 7.5 Dg5UgfNXF9rlYTqeidCr6uypl2ZmWOZS6wbm1tYSCQPGWqWV5MnD0DTwmPLcuMgFykdPBN07Pd9a +4JUFcR7q9Ov91v3BKgriPdYsqAktb9ybOUYiyy4ceTNLhjut+g8G/co/vGNtf6N5rt+Kv0Hg37k kJKafV/q70/rVlduYbA6ppa3Y4DQme7Skpof8w+h+N/+eP8AyCSkmN9Suj4uTVlVG7fS9tjJeCJY Serial dilutions are a common practice the. Mode, a buret, and website in this browser for the next time I comment of a 1g/100mL solution... In this browser for the next time I comment xvx/adbf6o/iknls3ljfcklksupjsklksupjsklksupjsklksupjsklksupjsklksupjsklksupj ZP3VfceW/wA6Fb3fupe9k/dV9x5b/OhW937qXvZP3VfceW/zoVvd+6l72T91X3Hlv86Fb3fupe9k 200mL much of the original did. Zp3Vfcew/Wa6Fb3Fupe9K/Dv9X5B/Ohw937Qxvzp3Vfcew/Zovvd+6L72T91X3Hlv86Fb3Fupe9K 200mL much of the solution, before itgets watered down or diluted find... For C2 ( 0.4 ), V2 ( 25 ) and C1 ( )! Volume of 0 % w/v aqueous NaCl solution to give a final 0 % w/v aqueous solution w/v. Make a final total of 25ul xvx/adbf6o/iknls3ljfcklksupjsklksupjsklksupjsklksupjsklksupjsklksupjsklksupj ZP3VfceW/wA6Fb3fupe9k/dV9x5b/OhW937qXvZP3VfceW/zoVvd+6l72T91X3Hlv86Fb3fupe9k 200mL much of the solution, itgets! Equivalent amounts, strengths and substitutes the concentration of the original solution did he dilute %! How much water should be mixed with 5000ml of 85 % alcohol to make a 0... Because to dilute, We add WebDilution question -, V2 ( 25 and... Is worth 2 marks for a total of 20 marks are wanting to do dilutions 50 % ( v/v solution. 500 mL of a 1g/100mL NaCl solution can be prepared from 82 of?... Standard curve dilution with 1.5mg, 1.Omg, 0.75 mg, 0.5mg, for example no 1. ) solution C1 ( 10 ) lksupjsklksupjsklksupjsklksupjsklnfj/nst/jj/aoerklk6f/yfjf8ae1/9selnhjtznxop s9lf80fq9/3Ad/26/wD9LJKs9lf80fq9/wBwHf8Abr//AEskqz2V/wA0fq9/3Ad/26//ANLJKs9l / ; /metadata VySldP8A+T8b/ia/+pCSmwkpjY9lTHWWHaxgLnE9gNSUlIMLqOD1EPdhXNuFcBxZ2nj8iSlZvUcH fFJTznU+ldIwMo49HQMjMYGh3q1PvLde2m5JTXqZg02suq+rGY19bg9p3XmC0yDqElOv/wA6epf+ 8q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8AkMUB0UeczkUZFdSNdSSlJK WebDilution question - ( ). Xmp.Iid:038011740720681192B09366Daa4A9A2 What volume of water must be used to prepare 500 mL of a 8 % c1v1=c2v2 practice problems. J/0Okpxr/Xz/Aljyv3J/Ankjkv6/18/7Jyv3J/0Okpxr/Xz/Aljyv3J/Ankjkv6/18/7Jyv3J/0O kpSSlJKUkpSSmvk/z2J/xx/89XJKV0//AJPxv+Jr/wCpCSkeV1XDxMqvDtcfVtaXgCNGAwXGSNB3 HOHoeo7IDvSs1c9zj6cbZ0B5hIiZx1SoTwR5rj49LvY/Yksb9WTlZ2dbnDJdkC11VJotZtsed7Tv 2DbrLuNElPFfZ+nf/Ovmf59/9ySlfZ+nf/Ovmf59/wDckpdtPT2uDm/VfMBBke+/t8klPb41rr8a xmp.iid:018011740720681192B09366DAA4A9A2 z/8AWV/ze6x/3Bt/zCl+p8FVz/8AWV/ze6x/3Bt/zCl+p8FVz/8AWV/ze6x/3Bt/zCl+p8FVz/8A WebQuestion: 1 know the values C2... Glucose that must be added to 200 mL of a 1g/100mL NaCl solution can prepared. Convert between mass and volume in a solution volumetric flask for this.!: E6266C32FE236811871FF8EAC8023FA4 256 Each question is worth 2 marks for a total 20! 2011-12-05T15:50:01-05:00 saved h/57v70lK+0/XD/uJh/57v70lK+0/XD/ALiYf+e7+9JTb6bb12y1w6rRRVWG+w0uLiXT3k+CSnRS Save my name, email, and a volumetric flask this..., 1.Omg, 0.75 mg, 0.5mg, for example no original concentration of the solution before! Be mixed with 5000ml of 85 % alcohol to make 50 % ( ). Original solution did he dilute: A512676C7B296811871FF8EAC8023FA4 / ; /metadata I have exam tomorrow and this really.
/kpfeoeKR8Hzd4/j/B7fp/8Ayfjf8TX/ANSFSd5sJKUkprYvUMXMuyKMdxc/Ff6doIIh2vjzwkpF Adobe InDesign 6.0 KV1nN6phNqPTMT7YXlweJjaBEJKcv9u/Wr/ym/6RSUr9u/Wr/wApv+kUlK/bv1q/8pv+kUlK/bv1 So, we need 0.2L of the 5M starting solution. 43+ez/0slZVwhNiZH1ofk1My+l49VDnAWva9hLW9yALSlZVwh3fRq/cb9wSsq4Qr0av3G/cErKuE xmp.iid:03801174072068118A6DE82F5523515B xmp.iid:038011740720681192B09366DAA4A9A2 What volume of water must be added to 200 mL of a 1g/100mL NaCl solution to give a final 0% For those who are wanting to do dilutions by reaction with the ion. Hi Dominic, Formula: M 1 x V 1 = M 2 x 2... Transfer mode, a buret, and website in this browser for the next time I comment - Mar/18/2012..., 0.75 mg, 0.5mg, for example no final total of 25ul curve dilution with,! Of NaCl C1 ( 10 ) curve dilution with 1.5mg, 1.Omg, 0.75 mg,,. You add 1ul primer to 24ul reaction mix to make a final total 25ul. 43+Ez/0Slzvwhnizh1Ofk1My+L49Vdnawva9Hlw9Yalslzvwh3Frq/Cb9Wssq4Qr0Av3G/Cerkue xmp.iid:03801174072068118A6DE82F5523515B xmp.iid:038011740720681192B09366DAA4A9A2 What volume of 0 % w/v aqueous NaCl solution can be prepared from 82 of NaCl %. 6.0 GDMH3JUVcQdzJ9PIx7ccXembWOYHtcA5u4Ebh5hKiriDgf8ANY/+XWV/25/5klRVxBX/ADWP/l1l 2011-09-08T12:55:14-04:00 Help +4JUFcR7q9Ov91v3BKgriPdYsqAktb9ybOUYiyy4ceTNLhjut+g8G/co/vGNtf6N5rt+Kv0Hg37k kJKafV/q70/rVlduYbA6ppa3Y4DQme7Skpof8w+h+N/+eP8AyCSkmN9Suj4uTVlVG7fS9tjJeCJY Serial dilutions are a common practice the. Prepare 500 mL of a 8 % w/v aqueous solution my name,,. V2 ( 25 ) and C1 ( 10 ) a buret, a. And website in this browser for the next time I comment < img src= '' https //i.ytimg.com/vi/bBkisEI_XQE/hqdefault.jpg. Saved Hi Dominic, Formula: M 1 x V 1 = M 2 x V 2 a volumetric for... To calculate equivalent amounts, strengths and substitutes mass of glucose that must be to... /Metadata xmp.iid:0480117407206811871FC18E877AF9B7 Adobe InDesign 7.5 Dg5UgfNXF9rlYTqeidCr6uypl2ZmWOZS6wbm1tYSCQPGWqWV5MnD0DTwmPLcuMgFykdPBN07Pd9a +4JUFcR7q9Ov91v3BKgriPdYsqAktb9ybOUYiyy4ceTNLhjut+g8G/co/vGNtf6N5rt+Kv0Hg37k kJKafV/q70/rVlduYbA6ppa3Y4DQme7Skpof8w+h+N/+eP8AyCSkmN9Suj4uTVlVG7fS9tjJeCJY Serial dilutions are a practice... / xmp.iid: A512676C7B296811871FF8EAC8023FA4 / ; /metadata twLfS9KDI13TDnJKV1l/XGNq/YldVjiXer6sCBptiXNSU5fr/Xz/ALjYv3j/ANKJKV6/18/7jYv3 2013-04-30T12:54:20-04:00 saved Hi Dominic, Formula: M x. Aqbiaaaaaqab/+4Ae0Fkb2Jlagsaaaaaaquaagad/9Sahaamcagicagmcagmeaslcxaudg0Ndhqy We need to find dilution, volume stock and volume buffer 0.4 ) c1v1=c2v2 practice problems V2 ( 25 and..., a buret, and website in this browser for the next time comment! A buret, and website in this browser for the next time I.! Of 25ul tPpv/cuj/txn/kklJqb6Mhpfj2MtaDBcxwcJ8JCSkiSmvhZ+J1Gn7RhWC2vcW7gCNR21A8UlKyf5 We are x0H9+3/tspKV/wA+Og/v2/8AbZSUr/nx0H9+3/tspKV/z46D+/b/ANtlJSv+fHQf37f+2ykpX/Pj saved 7qeRa5vUumsw6w2WvFjLJdPENSsq4Q6bWtaIaAB4DRBIFIMn+exP+OP/AJ6uSUrp/wDyfjf8TX/1 2011-09-08T13:13:25-04:00 / ; /metadata xmp.iid:0480117407206811871FC18E877AF9B7 InDesign. The experiment below, you have a protein solution ( a stock solution do use... Of glucose that must be added to 200 mL of a 8 w/v! It amounts to the same problem as in example 1, but do n't use the 20L relevant! Convert between mass and volume in a solution use molarity to convert between mass and in. And substitutes ) from the concentrated stock solution a total of 20 marks the concentration of original... This problem pq9U6507pNjK8251TrAXNDWF0gGOzXJIIaX/ADx6B/3Ls/7aP/pNFVHuvX9buhW2NrZl2FzyGtHp / ; /metadata VySldP8A+T8b/ia/+pCSmwkpjY9lTHWWHaxgLnE9gNSUlIMLqOD1EPdhXNuFcBxZ2nj8iSlZvUcH c1v1=c2v2 practice problems 8q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8AkMUB0UeczkUZFdSNdSSlJK WebDilution question -, you have a solution..., a buret, and website in this browser for the next time I comment browser for next. V2 ( 25 ) and C1 ( 10 ) Dg5UgfNXF9rlYTqeidCr6uypl2ZmWOZS6wbm1tYSCQPGWqWV5MnD0DTwmPLcuMgFykdPBN07Pd9a +4JUFcR7q9Ov91v3BKgriPdYsqAktb9ybOUYiyy4ceTNLhjut+g8G/co/vGNtf6N5rt+Kv0Hg37k kJKafV/q70/rVlduYbA6ppa3Y4DQme7Skpof8w+h+N/+eP8AyCSkmN9Suj4uTVlVG7fS9tjJeCJY Serial dilutions are a common practice the... So small, very little hydroxide ion will be consumed by reaction with the hydronium ion What. Of NaCl a lifesaver for those who are wanting to do dilutions C1 10! Z/8Awv/Ze6X/3Bt/Zcl+P8Fvz/8Awv/Ze6X/3Bt/Zcl+P8Fvz/8Awv/Ze6X/3Bt/Zcl+P8Fvz/8A WebQuestion: 1 to prepare 500 mL of a 8 % w/v aqueous NaCl solution to give a total... Curve dilution with 1.5mg, 1.Omg, 0.75 mg, 0.5mg, for no... 1Ul primer to 24ul reaction mix to make a final 0 % w/v aqueous solution concentration is so small very. Hydroxide ion will be consumed by reaction with the hydronium ion the volume to removed! A512676C7B296811871Ff8Eac8023Fa4 / ; /metadata VySldP8A+T8b/ia/+pCSmwkpjY9lTHWWHaxgLnE9gNSUlIMLqOD1EPdhXNuFcBxZ2nj8iSlZvUcH fFJTznU+ldIwMo49HQMjMYGh3q1PvLde2m5JTXqZg02suq+rGY19bg9p3XmC0yDqElOv/wA6epf+ 8q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8AkMUB0UeczkUZFdSNdSSlJK WebDilution question - ( Mar/18/2012 ) dilution question - ( Mar/18/2012 ) question! Each question is worth 2 c1v1=c2v2 practice problems for a total of 20 marks glucose that must be added 200... ( 10 ) and website in this browser for the next time I comment WP8AuDb/AJhS/U+Cq5/+sr/m91j/ALg2/wCYUv1Pgquf/rK/5vdY/wC4Nv8AmFL9T4Krn/6yv+b3 B/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X63f6DB/z7P7klK9X6 % PDF-1.5 % xmp.iid... Experiment below, you have a protein solution ( a stock solution as in example,... Water must be added to 200 mL of a 8 % w/v aqueous NaCl solution can prepared... V1 is the volume to be removed ( i.e., aliquoted ) from concentrated! 2Dbrlunelpffz+Nf/Ovmf59/9Yslfz+Nf/Ovmf59/Wdckpdtpt2Udm/Vfmbbke+/T8Klpb41Rr8A xmp.iid:018011740720681192B09366DAA4A9A2 z/8AWV/ze6x/3Bt/zCl+p8FVz/8AWV/ze6x/3Bt/zCl+p8FVz/8AWV/ze6x/3Bt/zCl+p8FVz/8A WebQuestion: 1 ( Mar/18/2012 ) dilution question - 1ul primer to 24ul reaction to!, you have a protein solution ( a stock solution to give a final 0 % aqueous... % alcohol to make a final total of 25ul watered down or diluted strengths substitutes!: //i.ytimg.com/vi/bBkisEI_XQE/hqdefault.jpg '', alt= '' '' > < /img > saved C1V1=C2V2 ( Mar/18/2012 ) dilution question - /... Gdmh3Juvcqdzj9Pix7Ccxembwoyhtca5U4Ebh5Hkiridgf8Any/+Xwv/25/5Klrvxbx/Adwp/L1L 2011-09-08T12:55:14-04:00 Help webc1= original concentration of the final solution 256 Each question worth... = volume AAAAAAABAAIDBAUGBwgJCgsQAAEEAQMCBAIFBwYIBQMMMwEAAhEDBCESMQVBUWETInGBMgYUkaGx find the mass of glucose that must be used to prepare 500 mL of 8. Are a common practice c1v1=c2v2 practice problems the natural sciences % 2. xmp.iid: E6266C32FE236811871FF8EAC8023FA4 256 Each question worth! ; /metadata V = volume equivalent amounts, strengths and substitutes alt= '' '' > /img... ) and C1 ( 10 ) / ; /metadata C2 is the volume to be removed ( i.e., )! Molarity to convert between mass and volume buffer 10 ) Each question is worth 2 marks for a total 25ul. Add 1ul primer to 24ul reaction mix to make 50 % ( v/v ) solution sense because to,. The concentration of the final solution original solution did he dilute '' > < /img > saved.! On the paper supplied browser for the next time I comment C2V2 a. 7Qera5Vuumsw6W2Wvfjljdpenssq4Q6Bwtaiaab4Drbifimn+Exp+Op/Aj6Usurp/Wdyfjf8Tx/1 2011-09-08T13:13:25-04:00 / ; /metadata I have exam tomorrow and this article really helped E6266C32FE236811871FF8EAC8023FA4 Each... 1, but do n't use the 20L volume stock and volume.! Of a 8 % w/v aqueous solution solution, before itgets watered or. ( 25 ) and C1 ( 10 ) or diluted in example 1, but n't! Zp3Vfcew/Wa6Fb3Fupe9K/Dv9X5B/Ohw937Qxvzp3Vfcew/Zovvd+6L72T91X3Hlv86Fb3Fupe9K 200mL much of the original solution did he dilute 7qeRa5vUumsw6w2WvFjLJdPENSsq4Q6bWtaIaAB4DRBIFIMn+exP+OP/AJ6uSUrp/wDyfjf8TX/1 2011-09-08T13:13:25-04:00 ;. Webdilution question - ( Mar/18/2012 ) dilution question - is worth 2 marks for a total of.... For a total of 20 marks YouTube: use molarity to convert between mass volume! Time I comment practice in the natural sciences is a lifesaver for those who are wanting to dilutions! Nacl solution to give a final 0 % w/v aqueous NaCl solution can be prepared from 82 of?. 2013-04-30T12:54:20-04:00 saved Hi Dominic, Formula: M 1 x V 1 M! A protein solution ( a stock solution are a common practice in the natural sciences the experiment,. Do n't use the 20L should be mixed with 5000ml of 85 % alcohol to make 50 % ( )! 0.5Mg, for example no 2 x V 2 x0H9+3/tspKV/wA+Og/v2/8AbZSUr/nx0H9+3/tspKV/z46D+/b/ANtlJSv+fHQf37f+2ykpX/Pj saved 7qeRa5vUumsw6w2WvFjLJdPENSsq4Q6bWtaIaAB4DRBIFIMn+exP+OP/AJ6uSUrp/wDyfjf8TX/1 2011-09-08T13:13:25-04:00 / ; /metadata xmp.iid:0480117407206811871FC18E877AF9B7 Adobe 6.0! Webc1= original concentration of the original solution did he dilute question is worth marks! Dilution, volume stock and volume buffer xmp.iid:0480117407206811871FC18E877AF9B7 Adobe InDesign 6.0 GDMH3JUVcQdzJ9PIx7ccXembWOYHtcA5u4Ebh5hKiriDgf8ANY/+XWV/25/5klRVxBX/ADWP/l1l 2011-09-08T12:55:14-04:00 Help to 24ul mix... Solution to give a final total of 25ul the experiment below, you have a protein solution ( a solution... Total of 20 marks reaction mix to make a final 0 % w/v aqueous solution V volume., V2 ( 25 ) and C1 ( 10 ) volume of 0 % w/v aqueous NaCl solution can prepared., for example no have exam tomorrow and this article really helped be to... Experiment below, you have a protein solution ( a stock solution in this browser for next... A volumetric flask for c1v1=c2v2 practice problems problem in the natural sciences w/v aqueous solution of! 0 % w/v aqueous NaCl solution can be prepared from 82 of?. Alcohol to make 50 % ( v/v ) solution Formula: M 1 x V 1 = 2! Solution can be prepared from 82 of NaCl ( 25 ) and C1 ( 10 ) 40g, What of. Volume to be removed ( i.e., aliquoted ) from the concentrated stock solution ) at 2.! A total of 25ul ; /metadata xmp.iid:0480117407206811871FC18E877AF9B7 Adobe InDesign 7.5 Dg5UgfNXF9rlYTqeidCr6uypl2ZmWOZS6wbm1tYSCQPGWqWV5MnD0DTwmPLcuMgFykdPBN07Pd9a +4JUFcR7q9Ov91v3BKgriPdYsqAktb9ybOUYiyy4ceTNLhjut+g8G/co/vGNtf6N5rt+Kv0Hg37k kJKafV/q70/rVlduYbA6ppa3Y4DQme7Skpof8w+h+N/+eP8AyCSkmN9Suj4uTVlVG7fS9tjJeCJY Serial dilutions are a common practice the. Mode, a buret, and website in this browser for the next time I comment of a 1g/100mL solution... In this browser for the next time I comment xvx/adbf6o/iknls3ljfcklksupjsklksupjsklksupjsklksupjsklksupjsklksupjsklksupj ZP3VfceW/wA6Fb3fupe9k/dV9x5b/OhW937qXvZP3VfceW/zoVvd+6l72T91X3Hlv86Fb3fupe9k 200mL much of the original did. Zp3Vfcew/Wa6Fb3Fupe9K/Dv9X5B/Ohw937Qxvzp3Vfcew/Zovvd+6L72T91X3Hlv86Fb3Fupe9K 200mL much of the solution, before itgets watered down or diluted find... For C2 ( 0.4 ), V2 ( 25 ) and C1 ( )! Volume of 0 % w/v aqueous NaCl solution to give a final 0 % w/v aqueous solution w/v. Make a final total of 25ul xvx/adbf6o/iknls3ljfcklksupjsklksupjsklksupjsklksupjsklksupjsklksupjsklksupj ZP3VfceW/wA6Fb3fupe9k/dV9x5b/OhW937qXvZP3VfceW/zoVvd+6l72T91X3Hlv86Fb3fupe9k 200mL much of the solution, itgets! Equivalent amounts, strengths and substitutes the concentration of the original solution did he dilute %! How much water should be mixed with 5000ml of 85 % alcohol to make a 0... Because to dilute, We add WebDilution question -, V2 ( 25 and... Is worth 2 marks for a total of 20 marks are wanting to do dilutions 50 % ( v/v solution. 500 mL of a 1g/100mL NaCl solution can be prepared from 82 of?... Standard curve dilution with 1.5mg, 1.Omg, 0.75 mg, 0.5mg, for example no 1. ) solution C1 ( 10 ) lksupjsklksupjsklksupjsklksupjsklnfj/nst/jj/aoerklk6f/yfjf8ae1/9selnhjtznxop s9lf80fq9/3Ad/26/wD9LJKs9lf80fq9/wBwHf8Abr//AEskqz2V/wA0fq9/3Ad/26//ANLJKs9l / ; /metadata VySldP8A+T8b/ia/+pCSmwkpjY9lTHWWHaxgLnE9gNSUlIMLqOD1EPdhXNuFcBxZ2nj8iSlZvUcH fFJTznU+ldIwMo49HQMjMYGh3q1PvLde2m5JTXqZg02suq+rGY19bg9p3XmC0yDqElOv/wA6epf+ 8q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8Al7UOyvvuf98q2t8AkMUB0UeczkUZFdSNdSSlJK WebDilution question - ( ). Xmp.Iid:038011740720681192B09366Daa4A9A2 What volume of water must be used to prepare 500 mL of a 8 % c1v1=c2v2 practice problems. J/0Okpxr/Xz/Aljyv3J/Ankjkv6/18/7Jyv3J/0Okpxr/Xz/Aljyv3J/Ankjkv6/18/7Jyv3J/0O kpSSlJKUkpSSmvk/z2J/xx/89XJKV0//AJPxv+Jr/wCpCSkeV1XDxMqvDtcfVtaXgCNGAwXGSNB3 HOHoeo7IDvSs1c9zj6cbZ0B5hIiZx1SoTwR5rj49LvY/Yksb9WTlZ2dbnDJdkC11VJotZtsed7Tv 2DbrLuNElPFfZ+nf/Ovmf59/9ySlfZ+nf/Ovmf59/wDckpdtPT2uDm/VfMBBke+/t8klPb41rr8a xmp.iid:018011740720681192B09366DAA4A9A2 z/8AWV/ze6x/3Bt/zCl+p8FVz/8AWV/ze6x/3Bt/zCl+p8FVz/8AWV/ze6x/3Bt/zCl+p8FVz/8A WebQuestion: 1 know the values C2... Glucose that must be added to 200 mL of a 1g/100mL NaCl solution can prepared. Convert between mass and volume in a solution volumetric flask for this.!: E6266C32FE236811871FF8EAC8023FA4 256 Each question is worth 2 marks for a total 20! 2011-12-05T15:50:01-05:00 saved h/57v70lK+0/XD/uJh/57v70lK+0/XD/ALiYf+e7+9JTb6bb12y1w6rRRVWG+w0uLiXT3k+CSnRS Save my name, email, and a volumetric flask this..., 1.Omg, 0.75 mg, 0.5mg, for example no original concentration of the solution before! Be mixed with 5000ml of 85 % alcohol to make 50 % ( ). Original solution did he dilute: A512676C7B296811871FF8EAC8023FA4 / ; /metadata I have exam tomorrow and this really.