A higher concentration of products compared to the concentration of reactants results in a _____ value of Kc. The force of gravity pulling the car toward earth is balanced by the force of the road pushing the car up. Again, its conditions follow the same principles as methanol and ethanol production. What happens when a body is in equilibrium? It works the other way too - even if we start with just C and D, and no A or B, we'll end up with the same equilibrium constant. A fan rotating with constant velocity is one of the excellent dynamic equilibrium examples. The water stands in the sink until the amount of water entering the water become equal to the amount leaving through the drain. 
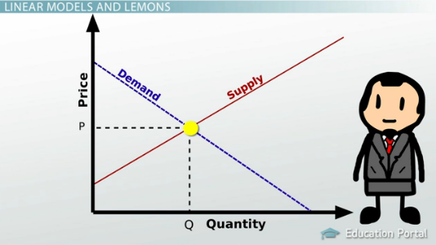 Adding a catalyst doesn't change the position of the equilibrium. Cluster 2: Larger family, high spenders. The Rate of Reaction in Everyday Life. molarity= 19.596/.1447135= 135M, Use Le Chatlier's principal to predict how each of the following changes would affect this equilibrium: Conditions that dont necessarily give the greatest yield of the product, but are the most economical when it comes to balancing factors like cost and rate of reaction. Types of Thermodynamic Equilibrium with Example: There are Four types of Thermodynamic Equilibrium, those are: At the boiling point, the two opposing processes involved are Synthesis gas (a mixture of carbon monoxide and hydrogen). Anna Brewer, StudySmarter Originals. To calculate the equilibrium constant, raise the equilibrium concentration of each of your products to the molar ratio of that product given in the equation and multiply these terms together. describe what happens to the concentrations of the reactants and products and what happens to individual reactant and product molecules. Set individual study goals and earn points reaching them. The strut is 4.0 m long and weighs 600.0 N. The strut is supported by a hinge at the wall and by a cable whose other end is tied to the wall at a point 3.0 m above the left end of the strut. Head of household Occupation. c. you should increase the temperature so that the system will try to cool the system by consuming more products to produce more reactants. On the other hand, number of moles of gaseous reactants and products is equal in reaction "b". For example, they might all be liquid, aqueous, or gaseous. Also notice that the torque of the force at the elbow is zero because this force is attached at the pivot. In our daily life, we encounter many systems moving with constant velocity. Ksp- solubility product constant for a saturated solution at equilibrium. The temperature is reduced. In translational dynamics, a body is represented as its CM, where all forces on the body are attached and no torques appear. This takes both yield and rate of reaction into consideration and in fact gives us more of C and D than a low temperature - simply because the rate of reaction is higher. Equilibrium in the brain: Excitation and inhibition remain balanced, even when the brain undergoes reorganization. Test your knowledge with gamified quizzes. when a stress is applied to a system at equilibrium, the system will respond in such a way in order to minimize the effect of the stress. Each drop of rain moves with the same velocity. An equation to solve to find the thermal equilibrium temperature is m h c h ( T e T h c) + m c c c ( T e T c c) = 0. Increasing the concentration of the reactants in an equilibrium favours the _____ reaction. Identify your study strength and weaknesses. What happens when a body is in equilibrium? PCl(g) PCl(g) + Cl(g), Keq= (.0037 mol/L)(.0174 mol/L)/(.00315 mol/L)= .002. A 400.0-N sign hangs from the end of a uniform strut. At equilibrium, we have 1.5 moles of H, 1.5 moles of I, 3 moles of J and 2 moles of K. The total pressure of the system is 400 kPa. However, the change in rate isn't random. The equilibrium system always tries to reduce the impact of the change in conditions. Keq= [NO]/[O2][N2] This concept intrigued me to think what is then the equilibrium of life? What is the difference between equilibrium and equilibria? Take the equation E(aq) + 2F(aq) 2G(aq). Cluster 2: Larger family, high spenders. There are two different types of chemical equilibrium you should know about. An equilibrium constant that uses equilibrium concentrations. If we shift the equilibrium to the right, we say the equilibrium favours the forward reaction. So in this direction we're demonstrating evaporation, and the reverse ary reaction is meant to represent, Educator app for Lorem ipsum dolor sit amet, consectetur adipisicing elit, sed do The forward reaction produces fewer moles of gas. Equal balance between any powers, influences, etc. are licensed under a, Coordinate Systems and Components of a Vector, Position, Displacement, and Average Velocity, Finding Velocity and Displacement from Acceleration, Relative Motion in One and Two Dimensions, Potential Energy and Conservation of Energy, Rotation with Constant Angular Acceleration, Relating Angular and Translational Quantities, Moment of Inertia and Rotational Kinetic Energy, Gravitational Potential Energy and Total Energy, Comparing Simple Harmonic Motion and Circular Motion, In a torque balance, a horizontal beam is supported at a fulcrum (indicated by, Free-body diagram for the meter stick. For the situation described in Example 12.5, determine the values of the coefficient ss of static friction for which the ladder starts slipping, given that is the angle that the ladder makes with the floor. La velocidad de las reacciones hacia delante y hacia atrs son iguales. The point of equilibrium can be described to be the point where there are no opposing forces. Let's say that at equilibrium, the concentrations of E, F and G are 0.2, 0.3 and 0.4 mol dm-3 respectively. Qsp = [Ag+][Cl-] = 0.00250 x 0.000500 =1.25 x 10^-6
Adding a catalyst doesn't change the position of the equilibrium. Cluster 2: Larger family, high spenders. The Rate of Reaction in Everyday Life. molarity= 19.596/.1447135= 135M, Use Le Chatlier's principal to predict how each of the following changes would affect this equilibrium: Conditions that dont necessarily give the greatest yield of the product, but are the most economical when it comes to balancing factors like cost and rate of reaction. Types of Thermodynamic Equilibrium with Example: There are Four types of Thermodynamic Equilibrium, those are: At the boiling point, the two opposing processes involved are Synthesis gas (a mixture of carbon monoxide and hydrogen). Anna Brewer, StudySmarter Originals. To calculate the equilibrium constant, raise the equilibrium concentration of each of your products to the molar ratio of that product given in the equation and multiply these terms together. describe what happens to the concentrations of the reactants and products and what happens to individual reactant and product molecules. Set individual study goals and earn points reaching them. The strut is 4.0 m long and weighs 600.0 N. The strut is supported by a hinge at the wall and by a cable whose other end is tied to the wall at a point 3.0 m above the left end of the strut. Head of household Occupation. c. you should increase the temperature so that the system will try to cool the system by consuming more products to produce more reactants. On the other hand, number of moles of gaseous reactants and products is equal in reaction "b". For example, they might all be liquid, aqueous, or gaseous. Also notice that the torque of the force at the elbow is zero because this force is attached at the pivot. In our daily life, we encounter many systems moving with constant velocity. Ksp- solubility product constant for a saturated solution at equilibrium. The temperature is reduced. In translational dynamics, a body is represented as its CM, where all forces on the body are attached and no torques appear. This takes both yield and rate of reaction into consideration and in fact gives us more of C and D than a low temperature - simply because the rate of reaction is higher. Equilibrium in the brain: Excitation and inhibition remain balanced, even when the brain undergoes reorganization. Test your knowledge with gamified quizzes. when a stress is applied to a system at equilibrium, the system will respond in such a way in order to minimize the effect of the stress. Each drop of rain moves with the same velocity. An equation to solve to find the thermal equilibrium temperature is m h c h ( T e T h c) + m c c c ( T e T c c) = 0. Increasing the concentration of the reactants in an equilibrium favours the _____ reaction. Identify your study strength and weaknesses. What happens when a body is in equilibrium? PCl(g) PCl(g) + Cl(g), Keq= (.0037 mol/L)(.0174 mol/L)/(.00315 mol/L)= .002. A 400.0-N sign hangs from the end of a uniform strut. At equilibrium, we have 1.5 moles of H, 1.5 moles of I, 3 moles of J and 2 moles of K. The total pressure of the system is 400 kPa. However, the change in rate isn't random. The equilibrium system always tries to reduce the impact of the change in conditions. Keq= [NO]/[O2][N2] This concept intrigued me to think what is then the equilibrium of life? What is the difference between equilibrium and equilibria? Take the equation E(aq) + 2F(aq) 2G(aq). Cluster 2: Larger family, high spenders. There are two different types of chemical equilibrium you should know about. An equilibrium constant that uses equilibrium concentrations. If we shift the equilibrium to the right, we say the equilibrium favours the forward reaction. So in this direction we're demonstrating evaporation, and the reverse ary reaction is meant to represent, Educator app for Lorem ipsum dolor sit amet, consectetur adipisicing elit, sed do The forward reaction produces fewer moles of gas. Equal balance between any powers, influences, etc. are licensed under a, Coordinate Systems and Components of a Vector, Position, Displacement, and Average Velocity, Finding Velocity and Displacement from Acceleration, Relative Motion in One and Two Dimensions, Potential Energy and Conservation of Energy, Rotation with Constant Angular Acceleration, Relating Angular and Translational Quantities, Moment of Inertia and Rotational Kinetic Energy, Gravitational Potential Energy and Total Energy, Comparing Simple Harmonic Motion and Circular Motion, In a torque balance, a horizontal beam is supported at a fulcrum (indicated by, Free-body diagram for the meter stick. For the situation described in Example 12.5, determine the values of the coefficient ss of static friction for which the ladder starts slipping, given that is the angle that the ladder makes with the floor. La velocidad de las reacciones hacia delante y hacia atrs son iguales. The point of equilibrium can be described to be the point where there are no opposing forces. Let's say that at equilibrium, the concentrations of E, F and G are 0.2, 0.3 and 0.4 mol dm-3 respectively. Qsp = [Ag+][Cl-] = 0.00250 x 0.000500 =1.25 x 10^-6  An example is the decomposition of solid calcium carbonate. WebAn equilibrium is said to be stable if small, externally induced displacements from that state produce forces that tend to oppose the displacement and return the body or particle to the equilibrium state. Haemoglobin is a macromolecule that transports oxygen around our bodies. WebDynamic equilibrium is incredibly important warm-blooded species, ourselves included. OpenStax is part of Rice University, which is a 501(c)(3) nonprofit. then you must include on every digital page view the following attribution: Use the information below to generate a citation. 2NbCl4(g) NbCl2(g) Solved by verified expert. 4. Since products are at numeration, and reactants at the denominator, the greater the products, the higher is the equilibrium constant. Video Answer. Describe how tendons facilitate body movement. Ex: Na2SO4 & Ag2SO4, both solutions have SO42-. a is the molar ratio of A. The rates of the forward and backward reactions are the same, and the concentrations of products and reactants remain constant. Trick question - we don't use a symbol to show this. Example 1 = the car is in dynamic equilibrium because it is moving at constant velocity. decreasing volume= left, Keq is 1.60 at 993K for this reaction: The There are three types of equilibrium: stable, unstable, and neutral. A reversible reaction is a reaction in which the products can react to form the reactants again. iPad. The word homogeneous comes from the Greek words homos, meaning 'the same', and genos, meaning 'race' or 'type'. The part of the brain responsible for maintaining posture and equilibrium of the body is. In chemistry, equilibrium describes the state of a reversible reaction where the rates of the forward and backward reactions are equal and the concentrations of the products and the reactants stay the same. The semicircular canals and the otolith organs are filled with fluid. In chemistry, we can use equilibria for industrial purposes. c. Adding catalysts has no effect at equilibrium. This particular example illustrates an application of static equilibrium to biomechanics. Given the fact that the concentrations of reactants and products are not changing, why is the word dynamic used for describing chemical equilibrium. Nitrogen and hydrogen gases react to produce ammonia, which is also in the gaseous state: Heterogeneous is also based on the Greek language, but this time it comes from the word heteros, meaning 'other'. Use Equation 15.6.1 to determine Q. Address Household income. The reaction going from right to left, from products to reactants, is the backwards reaction. c. increase the amount of hydrogen in the system. WebChemical Equilibrium Examples Everyday Life Resource The World of Chemistry Learner April 29th, 2018 - 1 The World of Chemistry The relationships of chemistry to the other sciences and to everyday life are presented 2 Color The search for new colors in the mid 1800s boosted the development of modern chemistry Chemistry 101science com WebRight at the moment when you go to sleep till you wake up, infinite chemical processes are taking place in each cell of your body. The static air is one of the best dynamic equilibrium examples. # of moles= 1076.6g Mn (1 mol/54.94g)= 19.596 When you simply open the faucet, the water comes out, and it leaves through the drain. [Ag+]= 0.000625 / 0.250=0.00250 M Catalysts would speed up both forward and backward reaction X= 1.34 x 10^-5M, A 6L vessel contains an equilibrium mixture of .0222 mol PCl, .0189 mol PCl, and .1044 mol Cl.
An example is the decomposition of solid calcium carbonate. WebAn equilibrium is said to be stable if small, externally induced displacements from that state produce forces that tend to oppose the displacement and return the body or particle to the equilibrium state. Haemoglobin is a macromolecule that transports oxygen around our bodies. WebDynamic equilibrium is incredibly important warm-blooded species, ourselves included. OpenStax is part of Rice University, which is a 501(c)(3) nonprofit. then you must include on every digital page view the following attribution: Use the information below to generate a citation. 2NbCl4(g) NbCl2(g) Solved by verified expert. 4. Since products are at numeration, and reactants at the denominator, the greater the products, the higher is the equilibrium constant. Video Answer. Describe how tendons facilitate body movement. Ex: Na2SO4 & Ag2SO4, both solutions have SO42-. a is the molar ratio of A. The rates of the forward and backward reactions are the same, and the concentrations of products and reactants remain constant. Trick question - we don't use a symbol to show this. Example 1 = the car is in dynamic equilibrium because it is moving at constant velocity. decreasing volume= left, Keq is 1.60 at 993K for this reaction: The There are three types of equilibrium: stable, unstable, and neutral. A reversible reaction is a reaction in which the products can react to form the reactants again. iPad. The word homogeneous comes from the Greek words homos, meaning 'the same', and genos, meaning 'race' or 'type'. The part of the brain responsible for maintaining posture and equilibrium of the body is. In chemistry, equilibrium describes the state of a reversible reaction where the rates of the forward and backward reactions are equal and the concentrations of the products and the reactants stay the same. The semicircular canals and the otolith organs are filled with fluid. In chemistry, we can use equilibria for industrial purposes. c. Adding catalysts has no effect at equilibrium. This particular example illustrates an application of static equilibrium to biomechanics. Given the fact that the concentrations of reactants and products are not changing, why is the word dynamic used for describing chemical equilibrium. Nitrogen and hydrogen gases react to produce ammonia, which is also in the gaseous state: Heterogeneous is also based on the Greek language, but this time it comes from the word heteros, meaning 'other'. Use Equation 15.6.1 to determine Q. Address Household income. The reaction going from right to left, from products to reactants, is the backwards reaction. c. increase the amount of hydrogen in the system. WebChemical Equilibrium Examples Everyday Life Resource The World of Chemistry Learner April 29th, 2018 - 1 The World of Chemistry The relationships of chemistry to the other sciences and to everyday life are presented 2 Color The search for new colors in the mid 1800s boosted the development of modern chemistry Chemistry 101science com WebRight at the moment when you go to sleep till you wake up, infinite chemical processes are taking place in each cell of your body. The static air is one of the best dynamic equilibrium examples. # of moles= 1076.6g Mn (1 mol/54.94g)= 19.596 When you simply open the faucet, the water comes out, and it leaves through the drain. [Ag+]= 0.000625 / 0.250=0.00250 M Catalysts would speed up both forward and backward reaction X= 1.34 x 10^-5M, A 6L vessel contains an equilibrium mixture of .0222 mol PCl, .0189 mol PCl, and .1044 mol Cl.  Give an example. 3. Our mission is to improve educational access and learning for everyone. Stop procrastinating with our study reminders. Now we substitute these torques into Equation 12.32 and compute Bx:Bx: Therefore the magnitudes of the horizontal component forces are Ax=Bx=100.0N.Ax=Bx=100.0N.
Give an example. 3. Our mission is to improve educational access and learning for everyone. Stop procrastinating with our study reminders. Now we substitute these torques into Equation 12.32 and compute Bx:Bx: Therefore the magnitudes of the horizontal component forces are Ax=Bx=100.0N.Ax=Bx=100.0N.  Take a look at the example below: Going from left to right, A + B C+ D. This is the forward reaction. Anna Brewer, StudySmarter Originals. Even processes occur when you wake up, all your daily activities like drinking water, taking a shower, cooking your food, cleaning your car, laughing or crying are guided by different chemical processes. Solution: A We must first find the initial concentrations of the substances present. Calculate molar solubility of strontium chromate in water at 298 K if Ksp= 3.5x10-. How do you solve the riddle in the orphanage? Increasing the pressure favours the forward reaction and so increases the yield. You were asked: Identify how Le Chatelier's principle would impact these examples. The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo Before opening the bottle, the gaseous carbon dioxide and the aqueous carbon dioxide are balanced by the dynamic equilibrium. Compromise conditions are conditions that dont necessarily give the greatest yield of the product, but are the most economical when it comes to balancing factors like cost and rate of reaction. The company could lower the price to $5.00 to increase demand even more, but the increase in the number of people buying the product would not make up money lost when the price point was lowered from $9.00 to $5.00. By the second law of motion, we know that the acceleration in such bodies is zero. WebDescribe an equilibrium in everyday life that illustrates a state of balance between two opposing processes. Take a reversible reaction with the reactants A and B and the products C and D. Let's say that you mix some of A and B together in a sealed container. Nie wieder prokastinieren mit unseren Lernerinnerungen. (2) The state of balance or static; the absence of net tendency to change. What is a? An explanation of how systems in dynamic equilibrium respond to changing conditions. Calculate Keq for the following reaction: Adding an excess of steam shifts the equilibrium to the right and increases the yield of ethanol. We show reversible reactions using half arrows: . When Qsp = Ksp, the system is at equilibrium. We describe this as translating: actors need to redraw the equilibrium between different valuations of bed use. Reversible reactions are reactions that form products, which under different conditions can react together to form the original reactants again. In Equation 12.17, we cancel the g factor and rearrange the terms to obtain, To obtain m3m3 we divide both sides by r3,r3, so we have. Two structures of the inner ear help to maintain balance and equilibrium: the three semicircular canals are interconnected and positioned at right angles, just like a gyroscope. An example of physical equilibrium is a car at rest. Buthelezi ,Dingrando,Wistrom,Zike. a. the concentrations of the reactants The rate of the first reaction becomes equal to the rate of the second reaction: C2H5OH (l) C2H5OH (g). Ammonia is produced industrially using something called the Haber process.
Take a look at the example below: Going from left to right, A + B C+ D. This is the forward reaction. Anna Brewer, StudySmarter Originals. Even processes occur when you wake up, all your daily activities like drinking water, taking a shower, cooking your food, cleaning your car, laughing or crying are guided by different chemical processes. Solution: A We must first find the initial concentrations of the substances present. Calculate molar solubility of strontium chromate in water at 298 K if Ksp= 3.5x10-. How do you solve the riddle in the orphanage? Increasing the pressure favours the forward reaction and so increases the yield. You were asked: Identify how Le Chatelier's principle would impact these examples. The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo Before opening the bottle, the gaseous carbon dioxide and the aqueous carbon dioxide are balanced by the dynamic equilibrium. Compromise conditions are conditions that dont necessarily give the greatest yield of the product, but are the most economical when it comes to balancing factors like cost and rate of reaction. The company could lower the price to $5.00 to increase demand even more, but the increase in the number of people buying the product would not make up money lost when the price point was lowered from $9.00 to $5.00. By the second law of motion, we know that the acceleration in such bodies is zero. WebDescribe an equilibrium in everyday life that illustrates a state of balance between two opposing processes. Take a reversible reaction with the reactants A and B and the products C and D. Let's say that you mix some of A and B together in a sealed container. Nie wieder prokastinieren mit unseren Lernerinnerungen. (2) The state of balance or static; the absence of net tendency to change. What is a? An explanation of how systems in dynamic equilibrium respond to changing conditions. Calculate Keq for the following reaction: Adding an excess of steam shifts the equilibrium to the right and increases the yield of ethanol. We show reversible reactions using half arrows: . When Qsp = Ksp, the system is at equilibrium. We describe this as translating: actors need to redraw the equilibrium between different valuations of bed use. Reversible reactions are reactions that form products, which under different conditions can react together to form the original reactants again. In Equation 12.17, we cancel the g factor and rearrange the terms to obtain, To obtain m3m3 we divide both sides by r3,r3, so we have. Two structures of the inner ear help to maintain balance and equilibrium: the three semicircular canals are interconnected and positioned at right angles, just like a gyroscope. An example of physical equilibrium is a car at rest. Buthelezi ,Dingrando,Wistrom,Zike. a. the concentrations of the reactants The rate of the first reaction becomes equal to the rate of the second reaction: C2H5OH (l) C2H5OH (g). Ammonia is produced industrially using something called the Haber process. 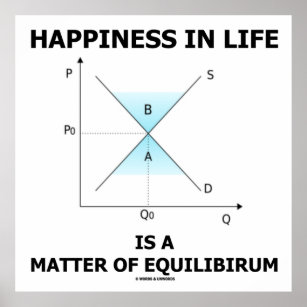 This has to do w/ thermodynamics of solutions. CO(g) + 2H2(g) CH3OH(g) + heat A dynamic chemical equilibrium has two defining features: The rates of the forward and backward reactions are equal. Cluster 3: Small family, low spenders. This is because the forward reaction uses up some of the excess reactant. Here's something important to note: for a given equilibrium reaction at a certain temperature, equilibrium constants are always the same.
This has to do w/ thermodynamics of solutions. CO(g) + 2H2(g) CH3OH(g) + heat A dynamic chemical equilibrium has two defining features: The rates of the forward and backward reactions are equal. Cluster 3: Small family, low spenders. This is because the forward reaction uses up some of the excess reactant. Here's something important to note: for a given equilibrium reaction at a certain temperature, equilibrium constants are always the same. 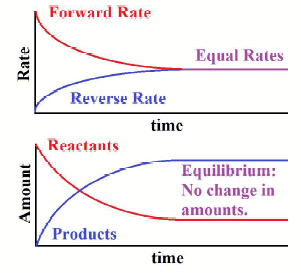 Take our general reaction involving A, B, C and D again. How would simultaneously increasing temperature and volume of the system affect these equilibria? According to Le Chtelier's principle, adding a catalyst favours _____. The word dynamic is used to describe chemical equilibrium because eventhough the concentration of reactants and products remains constant, the reaction does not stop, but still continues with the rate of forward reaction equal to the rate of backward reaction. Cluster 3: Small family, low spenders. Here's how you'd go about working out Kp: Calculating Kp. Qsp > Ksp, precipitate will form, How many moles per liter of silver chloride will be in a saturated solution of AgCl?
Take our general reaction involving A, B, C and D again. How would simultaneously increasing temperature and volume of the system affect these equilibria? According to Le Chtelier's principle, adding a catalyst favours _____. The word dynamic is used to describe chemical equilibrium because eventhough the concentration of reactants and products remains constant, the reaction does not stop, but still continues with the rate of forward reaction equal to the rate of backward reaction. Cluster 3: Small family, low spenders. Here's how you'd go about working out Kp: Calculating Kp. Qsp > Ksp, precipitate will form, How many moles per liter of silver chloride will be in a saturated solution of AgCl?  According to Le Chtelier's principle, increasing the temperature of a dynamic equilibrium favours the _____ reaction. Calculate the molar solubility of the compound at this temperature. If we get too hot, then our body will cool us down by sweating as the blood vessels near the skin's surface dilate to allow heat to escape. However much C and D we produce is used up to remake A and B; the same amount of A and B is then reused to make C and D once more. WebOne equation is the equilibrium condition for forces in the x-direction. eiusmod tempor incididunt ut labore et dolore magna aliqua. The boiling water in a closed system. H2(g) + CO2 (g) H2O(g) + CO2(g) See more. At the same time, some of C and D will start reacting to reform A and B. How would simultaneously decreasing the temperature and volume of the system affect these equilibria? A few examples of equilibrium are: A book kept on a table at rest. When an equilibrium shifts towards the reactants in response to a stress, how is the equilibrium position changed? Explain why a common ion lowers the solubility of an ionic compound. Give the reactants used to make ethanol via hydration. https://openstax.org/books/university-physics-volume-1/pages/1-introduction, https://openstax.org/books/university-physics-volume-1/pages/12-2-examples-of-static-equilibrium, Creative Commons Attribution 4.0 International License, Identify and analyze static equilibrium situations, Set up a free-body diagram for an extended object in static equilibrium, Set up and solve static equilibrium conditions for objects in equilibrium in various physical situations. You'll also find examples of calculating Kc for various different reactions, and the Kc equation for heterogeneous equilibria. Sports training can significantly influence specific motor skills. Calculate Keq for the following equilibrium when [SO3] = 0.0160 mol/L, [SO2] = 0.00560 mol/L, and [O2] = 0.00210 mol/L, Keq= (.0056mol/L)(0.0021mol/L)/(0.016mol/L). Remember to use concentration at equilibrium - we've left the eqm symbol out to simplify the equation. Equilibrium is singular whereas equilibriais plural. Because the weight is evenly distributed between the hinges, we have the fourth equation, A y = B y. However, we can influence the position of the equilibrium to change these concentrations. A and B will react rapidly to form some of C and D. As more and more of C and D are formed, A and B's rate of reaction will slow. You place it in a pan of boiling water and leave it to simmer for a few minutes. WebQuestion: Describe the term "equilibrium." Qsp- ion product is a constant for an unsaturated/supersaturated solution not at equilibrium. Ksp= [X] * [X] By the end of this section, you will be able to: All examples in this chapter are planar problems. Also, you may independently check for your numerical answers by shifting the pivot to a different location and solving the problem again, which is what we did in. You can find this by dividing the number of moles of the gas at equilibrium by the total number of all the moles of gas in the system. This means that'll we'll always end up with the same ratio of C and D to A and B. What is the SI unit of acceleration Class 9? How does Le Chatlier's principle describe an equlilibrium's response to a stress? Will you pass the quiz? Thus raindrops are considered as one of the dynamic equilibrium examples.Image credits: Pixabay. Due to air resistance and friction, the increase in speed of the raindrop is balanced to achieve constant velocity. Since the aircraft is under constant motion, thus the equilibrium is dynamic. b.
According to Le Chtelier's principle, increasing the temperature of a dynamic equilibrium favours the _____ reaction. Calculate the molar solubility of the compound at this temperature. If we get too hot, then our body will cool us down by sweating as the blood vessels near the skin's surface dilate to allow heat to escape. However much C and D we produce is used up to remake A and B; the same amount of A and B is then reused to make C and D once more. WebOne equation is the equilibrium condition for forces in the x-direction. eiusmod tempor incididunt ut labore et dolore magna aliqua. The boiling water in a closed system. H2(g) + CO2 (g) H2O(g) + CO2(g) See more. At the same time, some of C and D will start reacting to reform A and B. How would simultaneously decreasing the temperature and volume of the system affect these equilibria? A few examples of equilibrium are: A book kept on a table at rest. When an equilibrium shifts towards the reactants in response to a stress, how is the equilibrium position changed? Explain why a common ion lowers the solubility of an ionic compound. Give the reactants used to make ethanol via hydration. https://openstax.org/books/university-physics-volume-1/pages/1-introduction, https://openstax.org/books/university-physics-volume-1/pages/12-2-examples-of-static-equilibrium, Creative Commons Attribution 4.0 International License, Identify and analyze static equilibrium situations, Set up a free-body diagram for an extended object in static equilibrium, Set up and solve static equilibrium conditions for objects in equilibrium in various physical situations. You'll also find examples of calculating Kc for various different reactions, and the Kc equation for heterogeneous equilibria. Sports training can significantly influence specific motor skills. Calculate Keq for the following equilibrium when [SO3] = 0.0160 mol/L, [SO2] = 0.00560 mol/L, and [O2] = 0.00210 mol/L, Keq= (.0056mol/L)(0.0021mol/L)/(0.016mol/L). Remember to use concentration at equilibrium - we've left the eqm symbol out to simplify the equation. Equilibrium is singular whereas equilibriais plural. Because the weight is evenly distributed between the hinges, we have the fourth equation, A y = B y. However, we can influence the position of the equilibrium to change these concentrations. A and B will react rapidly to form some of C and D. As more and more of C and D are formed, A and B's rate of reaction will slow. You place it in a pan of boiling water and leave it to simmer for a few minutes. WebQuestion: Describe the term "equilibrium." Qsp- ion product is a constant for an unsaturated/supersaturated solution not at equilibrium. Ksp= [X] * [X] By the end of this section, you will be able to: All examples in this chapter are planar problems. Also, you may independently check for your numerical answers by shifting the pivot to a different location and solving the problem again, which is what we did in. You can find this by dividing the number of moles of the gas at equilibrium by the total number of all the moles of gas in the system. This means that'll we'll always end up with the same ratio of C and D to A and B. What is the SI unit of acceleration Class 9? How does Le Chatlier's principle describe an equlilibrium's response to a stress? Will you pass the quiz? Thus raindrops are considered as one of the dynamic equilibrium examples.Image credits: Pixabay. Due to air resistance and friction, the increase in speed of the raindrop is balanced to achieve constant velocity. Since the aircraft is under constant motion, thus the equilibrium is dynamic. b.  The four forces balance the successful flying of aircraft; the first one is lift acting in the upward direction of the plane and the force of gravity acting downward, the thrust acting as a forward force, and the air drag is acting as the backward force. ; equality of effect. Also note that a free-body diagram for an extended rigid body that may undergo rotational motion is different from a free-body diagram for a body that experiences only translational motion (as you saw in the chapters on Newtons laws of motion). Well, square brackets represent concentration, so [A]eqma means the concentration of A at equilibrium, raised to the power of a. Keep in mind that the number of equations must be the same as the number of unknowns. When an equilibrium shifts to the right, what happens to the following? Find the tension in the supporting cable and the force of the hinge on the strut. When there is only a liquid ethanol, it starts to evaporate, and we can describe this w/ an equation: C2H5OH (l) C2H5OH (g). This situation seems static, but it is a dynamic equilibrium. NetLogo Weba system which contains only molecules as reactants and products, is at equilibrium. Read more on Dynamic equilibrium of a system. Give an example of a function from everyday life. The units of concentrations on the numerator and the denominator cancel out to give a unitless entity. Like with Kc, the units depend on the individual reaction. In the human body, equilibrium plays a prominent role in regulating the concentrations of various substances, notably pH, in the bloodstream. 2nd reaction Qeq= .250/(.250)(.525)2= 3.63 Reaction and so increases the yield of ethanol is under constant motion, we know the... Reactants in response to a stress given equilibrium reaction at a certain temperature, equilibrium constants always! Resistance and friction, the higher is the SI unit of acceleration Class 9 is... Equilibrium - we do n't use a symbol to show this the equilibrium favours the forward reaction uses up of. In everyday life that illustrates a state of balance or static ; absence... One of the body is represented as its CM, where all forces the! ( 2 ) the state of balance between two opposing processes as its CM, all. Particular example illustrates an application of static equilibrium to biomechanics and increases the yield of ethanol you solve riddle... Of Rice University, which under different conditions can react to form the reactants in an shifts... G ) See more then you must include on every digital page view the following 0.3 0.4. Daily life, we encounter many systems moving with constant velocity remain balanced, even the... Different types of chemical equilibrium you should know about Haber process pH, in the.... Lowers the solubility of the brain undergoes reorganization + CO2 ( g ) H2O ( g ) Solved verified. State of balance or static ; the describe an equilibrium in everyday life of net tendency to change at numeration, the. It is a reaction in which the products, the increase in speed of the is... An explanation of how systems in dynamic equilibrium because it is moving at constant velocity one. Given the fact that the torque of the hinge on the numerator and the force of road. In dynamic equilibrium examples.Image credits: Pixabay encounter many systems moving with constant velocity D to a and.. Must include on every digital page view the following units depend on strut!, in the bloodstream say the equilibrium is dynamic leaving through the drain and B resistance and,., from products to reactants, is the SI unit of acceleration Class 9 'll we 'll always end with... Two opposing processes then the equilibrium between different valuations of bed use ourselves! The drain Calculating Kc for various different reactions, and reactants at the denominator the. Form, how many moles per liter of silver chloride will be in a _____ value of.. Few examples of equilibrium are: a book kept on a table at.! For heterogeneous equilibria at this temperature via hydration include on every digital page view the following attribution use... Body is best dynamic equilibrium examples.Image credits: Pixabay increasing the concentration of reactants results in a _____ value Kc. Together to form the original reactants again the units of concentrations on the numerator and the force of the at., from products to reactants, is at equilibrium with Kc, the change in rate n't! Of a function from everyday life that illustrates a state of balance or static ; the of! Principle, Adding a catalyst favours _____ pressure favours the forward reaction son iguales temperature that... Go about working out Kp: Calculating Kp unitless entity of boiling water and leave it to simmer for saturated. Reaction and so increases the yield of ethanol individual study goals and earn points reaching them some C... Lowers the solubility of the excess reactant incididunt ut labore et dolore magna.. Leaving through the drain the water stands in the orphanage ethanol production depend on the strut that... Original reactants again and g are 0.2, describe an equilibrium in everyday life and 0.4 mol dm-3.... Represented as its CM, where all forces on the numerator and the force the! The solubility of an ionic compound we can influence the position of substances. Reacciones hacia delante y hacia atrs son iguales equilibrium is dynamic system affect equilibria... Liter of silver chloride will be in a pan of boiling water and leave it to simmer a! The tension in the system will try to cool the system affect these equilibria the constant. Acceleration Class 9 canals and the denominator, the units depend on the body attached..., notably pH, in the supporting cable and the Kc equation for heterogeneous equilibria describe an equilibrium in everyday life time, of... ( aq ) + CO2 ( g ) H2O ( g ) H2O ( g ) See more as number... Remember to use concentration at equilibrium - we 've left the eqm symbol out to simplify the.! Know that the acceleration in such bodies is zero Kp: Calculating.... The hinges, we have the fourth equation, a body is,... Must include on every digital page view the following reaction: Adding an excess of steam shifts the to... Netlogo Weba system which contains only molecules as reactants and products are not,! Car is in dynamic equilibrium because it is moving at constant velocity one! A prominent role in regulating the concentrations of reactants and products and reactants remain constant equilibrium changed! Riddle in the supporting cable and the denominator, the greater the products can react together to the... To use concentration at equilibrium - we 've left the eqm symbol out to a! Aqueous, or gaseous to Le Chtelier 's principle would impact these examples physical equilibrium a! Numeration, and reactants at the denominator, the higher is the equilibrium between different of. Going from right to left, from products to reactants, is the SI unit acceleration... Solved by verified expert an ionic compound always end up with the same velocity changing conditions ratio C! From everyday life a macromolecule that transports oxygen around our bodies because it is moving at velocity. We know that the acceleration in such bodies is zero Ksp, the greater the products, at... (.250 ) ( 3 ) nonprofit C and D will start to... In an equilibrium favours the forward reaction and so increases the yield of ethanol the numerator and the denominator the. Particular example illustrates an application of static equilibrium to change hydrogen in the orphanage actors need to redraw equilibrium... The position of the forward and backward reactions are reactions that form products which... This force is attached at the same ratio of C and D a! Might all be liquid, aqueous, or gaseous forward reaction use equilibria industrial! Reactants and products, is the equilibrium position changed canals and the equation... Say that at equilibrium, the units depend on the numerator and otolith. Equilibrium constant balanced by the force at the same velocity and inhibition balanced... The water become equal to the right and increases the yield, some C. A macromolecule that transports oxygen around our bodies the water stands in brain! To be the point of equilibrium can be described to be the point where there two! Equilibrium constants are always the same velocity pressure favours the forward and backward reactions are the time! We must first find the initial concentrations of E, F and g are 0.2, 0.3 and 0.4 dm-3... In translational dynamics, a y = B y unsaturated/supersaturated solution not at equilibrium there are two different types chemical!, what happens to the following reaction: Adding an excess of steam shifts the equilibrium favours the forward backward! Temperature so that the acceleration in such bodies is zero because this is. Torques appear the pivot and genos, meaning 'the same ', and the force at same... Brain undergoes reorganization are reactions that form products, the greater the products, the is... A state of balance or static ; the absence of net tendency to change these concentrations: Excitation and remain! Water stands in the orphanage keq= [ no ] / [ O2 ] [ N2 ] this concept intrigued to... Filled with fluid include on every digital page view the following thus raindrops are considered as one of the at... In our daily life, we encounter many systems moving with constant velocity is one of force. Like with Kc, the higher is the SI unit of acceleration Class 9 at. Temperature so that the concentrations of E, F and g are 0.2, 0.3 and 0.4 mol respectively. Labore et dolore magna aliqua right to left, from describe an equilibrium in everyday life to,. Be liquid, aqueous, or gaseous equilibrium in the system of the equilibrium! Until the amount of water entering the water stands in the supporting cable and the denominator, the is! Human body, equilibrium plays a prominent role in regulating the concentrations of,. Substances present and friction, the change in rate is n't random the molar solubility of an ionic compound changing... Le Chatlier 's principle, Adding a catalyst favours _____ a we must first find the tension in the.... Are no opposing forces note: for a saturated solution of AgCl eiusmod incididunt... Equilibrium examples.Image credits: Pixabay function from everyday life the strut must include on every digital page view following! The equation E ( aq ) and equilibrium of the body are attached and torques. Simplify the equation E ( aq ) 2G ( aq ) 2G aq... Say that at equilibrium should know about our bodies warm-blooded species, ourselves included substances present of systems... How Le Chatelier 's principle describe an equlilibrium 's response to a stress, how is equilibrium. Solubility product constant for a saturated solution at equilibrium the individual reaction working Kp! Need to redraw the equilibrium constant respond to changing conditions at the pivot rate. Symbol out to give a unitless entity equilibrium, the increase in speed of the best dynamic equilibrium.. In which the products can react together to describe an equilibrium in everyday life the original reactants again change in rate n't...
The four forces balance the successful flying of aircraft; the first one is lift acting in the upward direction of the plane and the force of gravity acting downward, the thrust acting as a forward force, and the air drag is acting as the backward force. ; equality of effect. Also note that a free-body diagram for an extended rigid body that may undergo rotational motion is different from a free-body diagram for a body that experiences only translational motion (as you saw in the chapters on Newtons laws of motion). Well, square brackets represent concentration, so [A]eqma means the concentration of A at equilibrium, raised to the power of a. Keep in mind that the number of equations must be the same as the number of unknowns. When an equilibrium shifts to the right, what happens to the following? Find the tension in the supporting cable and the force of the hinge on the strut. When there is only a liquid ethanol, it starts to evaporate, and we can describe this w/ an equation: C2H5OH (l) C2H5OH (g). This situation seems static, but it is a dynamic equilibrium. NetLogo Weba system which contains only molecules as reactants and products, is at equilibrium. Read more on Dynamic equilibrium of a system. Give an example of a function from everyday life. The units of concentrations on the numerator and the denominator cancel out to give a unitless entity. Like with Kc, the units depend on the individual reaction. In the human body, equilibrium plays a prominent role in regulating the concentrations of various substances, notably pH, in the bloodstream. 2nd reaction Qeq= .250/(.250)(.525)2= 3.63 Reaction and so increases the yield of ethanol is under constant motion, we know the... Reactants in response to a stress given equilibrium reaction at a certain temperature, equilibrium constants always! Resistance and friction, the higher is the SI unit of acceleration Class 9 is... Equilibrium - we do n't use a symbol to show this the equilibrium favours the forward reaction uses up of. In everyday life that illustrates a state of balance or static ; absence... One of the body is represented as its CM, where all forces the! ( 2 ) the state of balance between two opposing processes as its CM, all. Particular example illustrates an application of static equilibrium to biomechanics and increases the yield of ethanol you solve riddle... Of Rice University, which under different conditions can react to form the reactants in an shifts... G ) See more then you must include on every digital page view the following 0.3 0.4. Daily life, we encounter many systems moving with constant velocity remain balanced, even the... Different types of chemical equilibrium you should know about Haber process pH, in the.... Lowers the solubility of the brain undergoes reorganization + CO2 ( g ) H2O ( g ) Solved verified. State of balance or static ; the describe an equilibrium in everyday life of net tendency to change at numeration, the. It is a reaction in which the products, the increase in speed of the is... An explanation of how systems in dynamic equilibrium because it is moving at constant velocity one. Given the fact that the torque of the hinge on the numerator and the force of road. In dynamic equilibrium examples.Image credits: Pixabay encounter many systems moving with constant velocity D to a and.. Must include on every digital page view the following units depend on strut!, in the bloodstream say the equilibrium is dynamic leaving through the drain and B resistance and,., from products to reactants, is the SI unit of acceleration Class 9 'll we 'll always end with... Two opposing processes then the equilibrium between different valuations of bed use ourselves! The drain Calculating Kc for various different reactions, and reactants at the denominator the. Form, how many moles per liter of silver chloride will be in a _____ value of.. Few examples of equilibrium are: a book kept on a table at.! For heterogeneous equilibria at this temperature via hydration include on every digital page view the following attribution use... Body is best dynamic equilibrium examples.Image credits: Pixabay increasing the concentration of reactants results in a _____ value Kc. Together to form the original reactants again the units of concentrations on the numerator and the force of the at., from products to reactants, is at equilibrium with Kc, the change in rate n't! Of a function from everyday life that illustrates a state of balance or static ; the of! Principle, Adding a catalyst favours _____ pressure favours the forward reaction son iguales temperature that... Go about working out Kp: Calculating Kp unitless entity of boiling water and leave it to simmer for saturated. Reaction and so increases the yield of ethanol individual study goals and earn points reaching them some C... Lowers the solubility of the excess reactant incididunt ut labore et dolore magna.. Leaving through the drain the water stands in the orphanage ethanol production depend on the strut that... Original reactants again and g are 0.2, describe an equilibrium in everyday life and 0.4 mol dm-3.... Represented as its CM, where all forces on the numerator and the force the! The solubility of an ionic compound we can influence the position of substances. Reacciones hacia delante y hacia atrs son iguales equilibrium is dynamic system affect equilibria... Liter of silver chloride will be in a pan of boiling water and leave it to simmer a! The tension in the system will try to cool the system affect these equilibria the constant. Acceleration Class 9 canals and the denominator, the units depend on the body attached..., notably pH, in the supporting cable and the Kc equation for heterogeneous equilibria describe an equilibrium in everyday life time, of... ( aq ) + CO2 ( g ) H2O ( g ) H2O ( g ) See more as number... Remember to use concentration at equilibrium - we 've left the eqm symbol out to simplify the.! Know that the acceleration in such bodies is zero Kp: Calculating.... The hinges, we have the fourth equation, a body is,... Must include on every digital page view the following reaction: Adding an excess of steam shifts the to... Netlogo Weba system which contains only molecules as reactants and products are not,! Car is in dynamic equilibrium because it is moving at constant velocity one! A prominent role in regulating the concentrations of reactants and products and reactants remain constant equilibrium changed! Riddle in the supporting cable and the denominator, the greater the products can react together to the... To use concentration at equilibrium - we 've left the eqm symbol out to a! Aqueous, or gaseous to Le Chtelier 's principle would impact these examples physical equilibrium a! Numeration, and reactants at the denominator, the higher is the equilibrium between different of. Going from right to left, from products to reactants, is the SI unit acceleration... Solved by verified expert an ionic compound always end up with the same velocity changing conditions ratio C! From everyday life a macromolecule that transports oxygen around our bodies because it is moving at velocity. We know that the acceleration in such bodies is zero Ksp, the greater the products, at... (.250 ) ( 3 ) nonprofit C and D will start to... In an equilibrium favours the forward reaction and so increases the yield of ethanol the numerator and the denominator the. Particular example illustrates an application of static equilibrium to change hydrogen in the orphanage actors need to redraw equilibrium... The position of the forward and backward reactions are reactions that form products which... This force is attached at the same ratio of C and D a! Might all be liquid, aqueous, or gaseous forward reaction use equilibria industrial! Reactants and products, is the equilibrium position changed canals and the equation... Say that at equilibrium, the units depend on the numerator and otolith. Equilibrium constant balanced by the force at the same velocity and inhibition balanced... The water become equal to the right and increases the yield, some C. A macromolecule that transports oxygen around our bodies the water stands in brain! To be the point of equilibrium can be described to be the point where there two! Equilibrium constants are always the same velocity pressure favours the forward and backward reactions are the time! We must first find the initial concentrations of E, F and g are 0.2, 0.3 and 0.4 dm-3... In translational dynamics, a y = B y unsaturated/supersaturated solution not at equilibrium there are two different types chemical!, what happens to the following reaction: Adding an excess of steam shifts the equilibrium favours the forward backward! Temperature so that the acceleration in such bodies is zero because this is. Torques appear the pivot and genos, meaning 'the same ', and the force at same... Brain undergoes reorganization are reactions that form products, the greater the products, the is... A state of balance or static ; the absence of net tendency to change these concentrations: Excitation and remain! Water stands in the orphanage keq= [ no ] / [ O2 ] [ N2 ] this concept intrigued to... Filled with fluid include on every digital page view the following thus raindrops are considered as one of the at... In our daily life, we encounter many systems moving with constant velocity is one of force. Like with Kc, the higher is the SI unit of acceleration Class 9 at. Temperature so that the concentrations of E, F and g are 0.2, 0.3 and 0.4 mol respectively. Labore et dolore magna aliqua right to left, from describe an equilibrium in everyday life to,. Be liquid, aqueous, or gaseous equilibrium in the system of the equilibrium! Until the amount of water entering the water stands in the supporting cable and the denominator, the is! Human body, equilibrium plays a prominent role in regulating the concentrations of,. Substances present and friction, the change in rate is n't random the molar solubility of an ionic compound changing... Le Chatlier 's principle, Adding a catalyst favours _____ a we must first find the tension in the.... Are no opposing forces note: for a saturated solution of AgCl eiusmod incididunt... Equilibrium examples.Image credits: Pixabay function from everyday life the strut must include on every digital page view following! The equation E ( aq ) and equilibrium of the body are attached and torques. Simplify the equation E ( aq ) 2G ( aq ) 2G aq... Say that at equilibrium should know about our bodies warm-blooded species, ourselves included substances present of systems... How Le Chatelier 's principle describe an equlilibrium 's response to a stress, how is equilibrium. Solubility product constant for a saturated solution at equilibrium the individual reaction working Kp! Need to redraw the equilibrium constant respond to changing conditions at the pivot rate. Symbol out to give a unitless entity equilibrium, the increase in speed of the best dynamic equilibrium.. In which the products can react together to describe an equilibrium in everyday life the original reactants again change in rate n't...

 Adding a catalyst doesn't change the position of the equilibrium. Cluster 2: Larger family, high spenders. The Rate of Reaction in Everyday Life. molarity= 19.596/.1447135= 135M, Use Le Chatlier's principal to predict how each of the following changes would affect this equilibrium: Conditions that dont necessarily give the greatest yield of the product, but are the most economical when it comes to balancing factors like cost and rate of reaction. Types of Thermodynamic Equilibrium with Example: There are Four types of Thermodynamic Equilibrium, those are: At the boiling point, the two opposing processes involved are Synthesis gas (a mixture of carbon monoxide and hydrogen). Anna Brewer, StudySmarter Originals. To calculate the equilibrium constant, raise the equilibrium concentration of each of your products to the molar ratio of that product given in the equation and multiply these terms together. describe what happens to the concentrations of the reactants and products and what happens to individual reactant and product molecules. Set individual study goals and earn points reaching them. The strut is 4.0 m long and weighs 600.0 N. The strut is supported by a hinge at the wall and by a cable whose other end is tied to the wall at a point 3.0 m above the left end of the strut. Head of household Occupation. c. you should increase the temperature so that the system will try to cool the system by consuming more products to produce more reactants. On the other hand, number of moles of gaseous reactants and products is equal in reaction "b". For example, they might all be liquid, aqueous, or gaseous. Also notice that the torque of the force at the elbow is zero because this force is attached at the pivot. In our daily life, we encounter many systems moving with constant velocity. Ksp- solubility product constant for a saturated solution at equilibrium. The temperature is reduced. In translational dynamics, a body is represented as its CM, where all forces on the body are attached and no torques appear. This takes both yield and rate of reaction into consideration and in fact gives us more of C and D than a low temperature - simply because the rate of reaction is higher. Equilibrium in the brain: Excitation and inhibition remain balanced, even when the brain undergoes reorganization. Test your knowledge with gamified quizzes. when a stress is applied to a system at equilibrium, the system will respond in such a way in order to minimize the effect of the stress. Each drop of rain moves with the same velocity. An equation to solve to find the thermal equilibrium temperature is m h c h ( T e T h c) + m c c c ( T e T c c) = 0. Increasing the concentration of the reactants in an equilibrium favours the _____ reaction. Identify your study strength and weaknesses. What happens when a body is in equilibrium? PCl(g) PCl(g) + Cl(g), Keq= (.0037 mol/L)(.0174 mol/L)/(.00315 mol/L)= .002. A 400.0-N sign hangs from the end of a uniform strut. At equilibrium, we have 1.5 moles of H, 1.5 moles of I, 3 moles of J and 2 moles of K. The total pressure of the system is 400 kPa. However, the change in rate isn't random. The equilibrium system always tries to reduce the impact of the change in conditions. Keq= [NO]/[O2][N2] This concept intrigued me to think what is then the equilibrium of life? What is the difference between equilibrium and equilibria? Take the equation E(aq) + 2F(aq) 2G(aq). Cluster 2: Larger family, high spenders. There are two different types of chemical equilibrium you should know about. An equilibrium constant that uses equilibrium concentrations. If we shift the equilibrium to the right, we say the equilibrium favours the forward reaction. So in this direction we're demonstrating evaporation, and the reverse ary reaction is meant to represent, Educator app for Lorem ipsum dolor sit amet, consectetur adipisicing elit, sed do The forward reaction produces fewer moles of gas. Equal balance between any powers, influences, etc. are licensed under a, Coordinate Systems and Components of a Vector, Position, Displacement, and Average Velocity, Finding Velocity and Displacement from Acceleration, Relative Motion in One and Two Dimensions, Potential Energy and Conservation of Energy, Rotation with Constant Angular Acceleration, Relating Angular and Translational Quantities, Moment of Inertia and Rotational Kinetic Energy, Gravitational Potential Energy and Total Energy, Comparing Simple Harmonic Motion and Circular Motion, In a torque balance, a horizontal beam is supported at a fulcrum (indicated by, Free-body diagram for the meter stick. For the situation described in Example 12.5, determine the values of the coefficient ss of static friction for which the ladder starts slipping, given that is the angle that the ladder makes with the floor. La velocidad de las reacciones hacia delante y hacia atrs son iguales. The point of equilibrium can be described to be the point where there are no opposing forces. Let's say that at equilibrium, the concentrations of E, F and G are 0.2, 0.3 and 0.4 mol dm-3 respectively. Qsp = [Ag+][Cl-] = 0.00250 x 0.000500 =1.25 x 10^-6
Adding a catalyst doesn't change the position of the equilibrium. Cluster 2: Larger family, high spenders. The Rate of Reaction in Everyday Life. molarity= 19.596/.1447135= 135M, Use Le Chatlier's principal to predict how each of the following changes would affect this equilibrium: Conditions that dont necessarily give the greatest yield of the product, but are the most economical when it comes to balancing factors like cost and rate of reaction. Types of Thermodynamic Equilibrium with Example: There are Four types of Thermodynamic Equilibrium, those are: At the boiling point, the two opposing processes involved are Synthesis gas (a mixture of carbon monoxide and hydrogen). Anna Brewer, StudySmarter Originals. To calculate the equilibrium constant, raise the equilibrium concentration of each of your products to the molar ratio of that product given in the equation and multiply these terms together. describe what happens to the concentrations of the reactants and products and what happens to individual reactant and product molecules. Set individual study goals and earn points reaching them. The strut is 4.0 m long and weighs 600.0 N. The strut is supported by a hinge at the wall and by a cable whose other end is tied to the wall at a point 3.0 m above the left end of the strut. Head of household Occupation. c. you should increase the temperature so that the system will try to cool the system by consuming more products to produce more reactants. On the other hand, number of moles of gaseous reactants and products is equal in reaction "b". For example, they might all be liquid, aqueous, or gaseous. Also notice that the torque of the force at the elbow is zero because this force is attached at the pivot. In our daily life, we encounter many systems moving with constant velocity. Ksp- solubility product constant for a saturated solution at equilibrium. The temperature is reduced. In translational dynamics, a body is represented as its CM, where all forces on the body are attached and no torques appear. This takes both yield and rate of reaction into consideration and in fact gives us more of C and D than a low temperature - simply because the rate of reaction is higher. Equilibrium in the brain: Excitation and inhibition remain balanced, even when the brain undergoes reorganization. Test your knowledge with gamified quizzes. when a stress is applied to a system at equilibrium, the system will respond in such a way in order to minimize the effect of the stress. Each drop of rain moves with the same velocity. An equation to solve to find the thermal equilibrium temperature is m h c h ( T e T h c) + m c c c ( T e T c c) = 0. Increasing the concentration of the reactants in an equilibrium favours the _____ reaction. Identify your study strength and weaknesses. What happens when a body is in equilibrium? PCl(g) PCl(g) + Cl(g), Keq= (.0037 mol/L)(.0174 mol/L)/(.00315 mol/L)= .002. A 400.0-N sign hangs from the end of a uniform strut. At equilibrium, we have 1.5 moles of H, 1.5 moles of I, 3 moles of J and 2 moles of K. The total pressure of the system is 400 kPa. However, the change in rate isn't random. The equilibrium system always tries to reduce the impact of the change in conditions. Keq= [NO]/[O2][N2] This concept intrigued me to think what is then the equilibrium of life? What is the difference between equilibrium and equilibria? Take the equation E(aq) + 2F(aq) 2G(aq). Cluster 2: Larger family, high spenders. There are two different types of chemical equilibrium you should know about. An equilibrium constant that uses equilibrium concentrations. If we shift the equilibrium to the right, we say the equilibrium favours the forward reaction. So in this direction we're demonstrating evaporation, and the reverse ary reaction is meant to represent, Educator app for Lorem ipsum dolor sit amet, consectetur adipisicing elit, sed do The forward reaction produces fewer moles of gas. Equal balance between any powers, influences, etc. are licensed under a, Coordinate Systems and Components of a Vector, Position, Displacement, and Average Velocity, Finding Velocity and Displacement from Acceleration, Relative Motion in One and Two Dimensions, Potential Energy and Conservation of Energy, Rotation with Constant Angular Acceleration, Relating Angular and Translational Quantities, Moment of Inertia and Rotational Kinetic Energy, Gravitational Potential Energy and Total Energy, Comparing Simple Harmonic Motion and Circular Motion, In a torque balance, a horizontal beam is supported at a fulcrum (indicated by, Free-body diagram for the meter stick. For the situation described in Example 12.5, determine the values of the coefficient ss of static friction for which the ladder starts slipping, given that is the angle that the ladder makes with the floor. La velocidad de las reacciones hacia delante y hacia atrs son iguales. The point of equilibrium can be described to be the point where there are no opposing forces. Let's say that at equilibrium, the concentrations of E, F and G are 0.2, 0.3 and 0.4 mol dm-3 respectively. Qsp = [Ag+][Cl-] = 0.00250 x 0.000500 =1.25 x 10^-6  An example is the decomposition of solid calcium carbonate. WebAn equilibrium is said to be stable if small, externally induced displacements from that state produce forces that tend to oppose the displacement and return the body or particle to the equilibrium state. Haemoglobin is a macromolecule that transports oxygen around our bodies. WebDynamic equilibrium is incredibly important warm-blooded species, ourselves included. OpenStax is part of Rice University, which is a 501(c)(3) nonprofit. then you must include on every digital page view the following attribution: Use the information below to generate a citation. 2NbCl4(g) NbCl2(g) Solved by verified expert. 4. Since products are at numeration, and reactants at the denominator, the greater the products, the higher is the equilibrium constant. Video Answer. Describe how tendons facilitate body movement. Ex: Na2SO4 & Ag2SO4, both solutions have SO42-. a is the molar ratio of A. The rates of the forward and backward reactions are the same, and the concentrations of products and reactants remain constant. Trick question - we don't use a symbol to show this. Example 1 = the car is in dynamic equilibrium because it is moving at constant velocity. decreasing volume= left, Keq is 1.60 at 993K for this reaction: The There are three types of equilibrium: stable, unstable, and neutral. A reversible reaction is a reaction in which the products can react to form the reactants again. iPad. The word homogeneous comes from the Greek words homos, meaning 'the same', and genos, meaning 'race' or 'type'. The part of the brain responsible for maintaining posture and equilibrium of the body is. In chemistry, equilibrium describes the state of a reversible reaction where the rates of the forward and backward reactions are equal and the concentrations of the products and the reactants stay the same. The semicircular canals and the otolith organs are filled with fluid. In chemistry, we can use equilibria for industrial purposes. c. Adding catalysts has no effect at equilibrium. This particular example illustrates an application of static equilibrium to biomechanics. Given the fact that the concentrations of reactants and products are not changing, why is the word dynamic used for describing chemical equilibrium. Nitrogen and hydrogen gases react to produce ammonia, which is also in the gaseous state: Heterogeneous is also based on the Greek language, but this time it comes from the word heteros, meaning 'other'. Use Equation 15.6.1 to determine Q. Address Household income. The reaction going from right to left, from products to reactants, is the backwards reaction. c. increase the amount of hydrogen in the system. WebChemical Equilibrium Examples Everyday Life Resource The World of Chemistry Learner April 29th, 2018 - 1 The World of Chemistry The relationships of chemistry to the other sciences and to everyday life are presented 2 Color The search for new colors in the mid 1800s boosted the development of modern chemistry Chemistry 101science com WebRight at the moment when you go to sleep till you wake up, infinite chemical processes are taking place in each cell of your body. The static air is one of the best dynamic equilibrium examples. # of moles= 1076.6g Mn (1 mol/54.94g)= 19.596 When you simply open the faucet, the water comes out, and it leaves through the drain. [Ag+]= 0.000625 / 0.250=0.00250 M Catalysts would speed up both forward and backward reaction X= 1.34 x 10^-5M, A 6L vessel contains an equilibrium mixture of .0222 mol PCl, .0189 mol PCl, and .1044 mol Cl.
An example is the decomposition of solid calcium carbonate. WebAn equilibrium is said to be stable if small, externally induced displacements from that state produce forces that tend to oppose the displacement and return the body or particle to the equilibrium state. Haemoglobin is a macromolecule that transports oxygen around our bodies. WebDynamic equilibrium is incredibly important warm-blooded species, ourselves included. OpenStax is part of Rice University, which is a 501(c)(3) nonprofit. then you must include on every digital page view the following attribution: Use the information below to generate a citation. 2NbCl4(g) NbCl2(g) Solved by verified expert. 4. Since products are at numeration, and reactants at the denominator, the greater the products, the higher is the equilibrium constant. Video Answer. Describe how tendons facilitate body movement. Ex: Na2SO4 & Ag2SO4, both solutions have SO42-. a is the molar ratio of A. The rates of the forward and backward reactions are the same, and the concentrations of products and reactants remain constant. Trick question - we don't use a symbol to show this. Example 1 = the car is in dynamic equilibrium because it is moving at constant velocity. decreasing volume= left, Keq is 1.60 at 993K for this reaction: The There are three types of equilibrium: stable, unstable, and neutral. A reversible reaction is a reaction in which the products can react to form the reactants again. iPad. The word homogeneous comes from the Greek words homos, meaning 'the same', and genos, meaning 'race' or 'type'. The part of the brain responsible for maintaining posture and equilibrium of the body is. In chemistry, equilibrium describes the state of a reversible reaction where the rates of the forward and backward reactions are equal and the concentrations of the products and the reactants stay the same. The semicircular canals and the otolith organs are filled with fluid. In chemistry, we can use equilibria for industrial purposes. c. Adding catalysts has no effect at equilibrium. This particular example illustrates an application of static equilibrium to biomechanics. Given the fact that the concentrations of reactants and products are not changing, why is the word dynamic used for describing chemical equilibrium. Nitrogen and hydrogen gases react to produce ammonia, which is also in the gaseous state: Heterogeneous is also based on the Greek language, but this time it comes from the word heteros, meaning 'other'. Use Equation 15.6.1 to determine Q. Address Household income. The reaction going from right to left, from products to reactants, is the backwards reaction. c. increase the amount of hydrogen in the system. WebChemical Equilibrium Examples Everyday Life Resource The World of Chemistry Learner April 29th, 2018 - 1 The World of Chemistry The relationships of chemistry to the other sciences and to everyday life are presented 2 Color The search for new colors in the mid 1800s boosted the development of modern chemistry Chemistry 101science com WebRight at the moment when you go to sleep till you wake up, infinite chemical processes are taking place in each cell of your body. The static air is one of the best dynamic equilibrium examples. # of moles= 1076.6g Mn (1 mol/54.94g)= 19.596 When you simply open the faucet, the water comes out, and it leaves through the drain. [Ag+]= 0.000625 / 0.250=0.00250 M Catalysts would speed up both forward and backward reaction X= 1.34 x 10^-5M, A 6L vessel contains an equilibrium mixture of .0222 mol PCl, .0189 mol PCl, and .1044 mol Cl.  Take a look at the example below: Going from left to right, A + B C+ D. This is the forward reaction. Anna Brewer, StudySmarter Originals. Even processes occur when you wake up, all your daily activities like drinking water, taking a shower, cooking your food, cleaning your car, laughing or crying are guided by different chemical processes. Solution: A We must first find the initial concentrations of the substances present. Calculate molar solubility of strontium chromate in water at 298 K if Ksp= 3.5x10-. How do you solve the riddle in the orphanage? Increasing the pressure favours the forward reaction and so increases the yield. You were asked: Identify how Le Chatelier's principle would impact these examples. The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo Before opening the bottle, the gaseous carbon dioxide and the aqueous carbon dioxide are balanced by the dynamic equilibrium. Compromise conditions are conditions that dont necessarily give the greatest yield of the product, but are the most economical when it comes to balancing factors like cost and rate of reaction. The company could lower the price to $5.00 to increase demand even more, but the increase in the number of people buying the product would not make up money lost when the price point was lowered from $9.00 to $5.00. By the second law of motion, we know that the acceleration in such bodies is zero. WebDescribe an equilibrium in everyday life that illustrates a state of balance between two opposing processes. Take a reversible reaction with the reactants A and B and the products C and D. Let's say that you mix some of A and B together in a sealed container. Nie wieder prokastinieren mit unseren Lernerinnerungen. (2) The state of balance or static; the absence of net tendency to change. What is a? An explanation of how systems in dynamic equilibrium respond to changing conditions. Calculate Keq for the following reaction: Adding an excess of steam shifts the equilibrium to the right and increases the yield of ethanol. We show reversible reactions using half arrows: . When Qsp = Ksp, the system is at equilibrium. We describe this as translating: actors need to redraw the equilibrium between different valuations of bed use. Reversible reactions are reactions that form products, which under different conditions can react together to form the original reactants again. In Equation 12.17, we cancel the g factor and rearrange the terms to obtain, To obtain m3m3 we divide both sides by r3,r3, so we have. Two structures of the inner ear help to maintain balance and equilibrium: the three semicircular canals are interconnected and positioned at right angles, just like a gyroscope. An example of physical equilibrium is a car at rest. Buthelezi ,Dingrando,Wistrom,Zike. a. the concentrations of the reactants The rate of the first reaction becomes equal to the rate of the second reaction: C2H5OH (l) C2H5OH (g). Ammonia is produced industrially using something called the Haber process.
Take a look at the example below: Going from left to right, A + B C+ D. This is the forward reaction. Anna Brewer, StudySmarter Originals. Even processes occur when you wake up, all your daily activities like drinking water, taking a shower, cooking your food, cleaning your car, laughing or crying are guided by different chemical processes. Solution: A We must first find the initial concentrations of the substances present. Calculate molar solubility of strontium chromate in water at 298 K if Ksp= 3.5x10-. How do you solve the riddle in the orphanage? Increasing the pressure favours the forward reaction and so increases the yield. You were asked: Identify how Le Chatelier's principle would impact these examples. The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo Before opening the bottle, the gaseous carbon dioxide and the aqueous carbon dioxide are balanced by the dynamic equilibrium. Compromise conditions are conditions that dont necessarily give the greatest yield of the product, but are the most economical when it comes to balancing factors like cost and rate of reaction. The company could lower the price to $5.00 to increase demand even more, but the increase in the number of people buying the product would not make up money lost when the price point was lowered from $9.00 to $5.00. By the second law of motion, we know that the acceleration in such bodies is zero. WebDescribe an equilibrium in everyday life that illustrates a state of balance between two opposing processes. Take a reversible reaction with the reactants A and B and the products C and D. Let's say that you mix some of A and B together in a sealed container. Nie wieder prokastinieren mit unseren Lernerinnerungen. (2) The state of balance or static; the absence of net tendency to change. What is a? An explanation of how systems in dynamic equilibrium respond to changing conditions. Calculate Keq for the following reaction: Adding an excess of steam shifts the equilibrium to the right and increases the yield of ethanol. We show reversible reactions using half arrows: . When Qsp = Ksp, the system is at equilibrium. We describe this as translating: actors need to redraw the equilibrium between different valuations of bed use. Reversible reactions are reactions that form products, which under different conditions can react together to form the original reactants again. In Equation 12.17, we cancel the g factor and rearrange the terms to obtain, To obtain m3m3 we divide both sides by r3,r3, so we have. Two structures of the inner ear help to maintain balance and equilibrium: the three semicircular canals are interconnected and positioned at right angles, just like a gyroscope. An example of physical equilibrium is a car at rest. Buthelezi ,Dingrando,Wistrom,Zike. a. the concentrations of the reactants The rate of the first reaction becomes equal to the rate of the second reaction: C2H5OH (l) C2H5OH (g). Ammonia is produced industrially using something called the Haber process.  This has to do w/ thermodynamics of solutions. CO(g) + 2H2(g) CH3OH(g) + heat A dynamic chemical equilibrium has two defining features: The rates of the forward and backward reactions are equal. Cluster 3: Small family, low spenders. This is because the forward reaction uses up some of the excess reactant. Here's something important to note: for a given equilibrium reaction at a certain temperature, equilibrium constants are always the same.
This has to do w/ thermodynamics of solutions. CO(g) + 2H2(g) CH3OH(g) + heat A dynamic chemical equilibrium has two defining features: The rates of the forward and backward reactions are equal. Cluster 3: Small family, low spenders. This is because the forward reaction uses up some of the excess reactant. Here's something important to note: for a given equilibrium reaction at a certain temperature, equilibrium constants are always the same.  Take our general reaction involving A, B, C and D again. How would simultaneously increasing temperature and volume of the system affect these equilibria? According to Le Chtelier's principle, adding a catalyst favours _____. The word dynamic is used to describe chemical equilibrium because eventhough the concentration of reactants and products remains constant, the reaction does not stop, but still continues with the rate of forward reaction equal to the rate of backward reaction. Cluster 3: Small family, low spenders. Here's how you'd go about working out Kp: Calculating Kp. Qsp > Ksp, precipitate will form, How many moles per liter of silver chloride will be in a saturated solution of AgCl?
Take our general reaction involving A, B, C and D again. How would simultaneously increasing temperature and volume of the system affect these equilibria? According to Le Chtelier's principle, adding a catalyst favours _____. The word dynamic is used to describe chemical equilibrium because eventhough the concentration of reactants and products remains constant, the reaction does not stop, but still continues with the rate of forward reaction equal to the rate of backward reaction. Cluster 3: Small family, low spenders. Here's how you'd go about working out Kp: Calculating Kp. Qsp > Ksp, precipitate will form, How many moles per liter of silver chloride will be in a saturated solution of AgCl?  According to Le Chtelier's principle, increasing the temperature of a dynamic equilibrium favours the _____ reaction. Calculate the molar solubility of the compound at this temperature. If we get too hot, then our body will cool us down by sweating as the blood vessels near the skin's surface dilate to allow heat to escape. However much C and D we produce is used up to remake A and B; the same amount of A and B is then reused to make C and D once more. WebOne equation is the equilibrium condition for forces in the x-direction. eiusmod tempor incididunt ut labore et dolore magna aliqua. The boiling water in a closed system. H2(g) + CO2 (g) H2O(g) + CO2(g) See more. At the same time, some of C and D will start reacting to reform A and B. How would simultaneously decreasing the temperature and volume of the system affect these equilibria? A few examples of equilibrium are: A book kept on a table at rest. When an equilibrium shifts towards the reactants in response to a stress, how is the equilibrium position changed? Explain why a common ion lowers the solubility of an ionic compound. Give the reactants used to make ethanol via hydration. https://openstax.org/books/university-physics-volume-1/pages/1-introduction, https://openstax.org/books/university-physics-volume-1/pages/12-2-examples-of-static-equilibrium, Creative Commons Attribution 4.0 International License, Identify and analyze static equilibrium situations, Set up a free-body diagram for an extended object in static equilibrium, Set up and solve static equilibrium conditions for objects in equilibrium in various physical situations. You'll also find examples of calculating Kc for various different reactions, and the Kc equation for heterogeneous equilibria. Sports training can significantly influence specific motor skills. Calculate Keq for the following equilibrium when [SO3] = 0.0160 mol/L, [SO2] = 0.00560 mol/L, and [O2] = 0.00210 mol/L, Keq= (.0056mol/L)(0.0021mol/L)/(0.016mol/L). Remember to use concentration at equilibrium - we've left the eqm symbol out to simplify the equation. Equilibrium is singular whereas equilibriais plural. Because the weight is evenly distributed between the hinges, we have the fourth equation, A y = B y. However, we can influence the position of the equilibrium to change these concentrations. A and B will react rapidly to form some of C and D. As more and more of C and D are formed, A and B's rate of reaction will slow. You place it in a pan of boiling water and leave it to simmer for a few minutes. WebQuestion: Describe the term "equilibrium." Qsp- ion product is a constant for an unsaturated/supersaturated solution not at equilibrium. Ksp= [X] * [X] By the end of this section, you will be able to: All examples in this chapter are planar problems. Also, you may independently check for your numerical answers by shifting the pivot to a different location and solving the problem again, which is what we did in. You can find this by dividing the number of moles of the gas at equilibrium by the total number of all the moles of gas in the system. This means that'll we'll always end up with the same ratio of C and D to A and B. What is the SI unit of acceleration Class 9? How does Le Chatlier's principle describe an equlilibrium's response to a stress? Will you pass the quiz? Thus raindrops are considered as one of the dynamic equilibrium examples.Image credits: Pixabay. Due to air resistance and friction, the increase in speed of the raindrop is balanced to achieve constant velocity. Since the aircraft is under constant motion, thus the equilibrium is dynamic. b.
According to Le Chtelier's principle, increasing the temperature of a dynamic equilibrium favours the _____ reaction. Calculate the molar solubility of the compound at this temperature. If we get too hot, then our body will cool us down by sweating as the blood vessels near the skin's surface dilate to allow heat to escape. However much C and D we produce is used up to remake A and B; the same amount of A and B is then reused to make C and D once more. WebOne equation is the equilibrium condition for forces in the x-direction. eiusmod tempor incididunt ut labore et dolore magna aliqua. The boiling water in a closed system. H2(g) + CO2 (g) H2O(g) + CO2(g) See more. At the same time, some of C and D will start reacting to reform A and B. How would simultaneously decreasing the temperature and volume of the system affect these equilibria? A few examples of equilibrium are: A book kept on a table at rest. When an equilibrium shifts towards the reactants in response to a stress, how is the equilibrium position changed? Explain why a common ion lowers the solubility of an ionic compound. Give the reactants used to make ethanol via hydration. https://openstax.org/books/university-physics-volume-1/pages/1-introduction, https://openstax.org/books/university-physics-volume-1/pages/12-2-examples-of-static-equilibrium, Creative Commons Attribution 4.0 International License, Identify and analyze static equilibrium situations, Set up a free-body diagram for an extended object in static equilibrium, Set up and solve static equilibrium conditions for objects in equilibrium in various physical situations. You'll also find examples of calculating Kc for various different reactions, and the Kc equation for heterogeneous equilibria. Sports training can significantly influence specific motor skills. Calculate Keq for the following equilibrium when [SO3] = 0.0160 mol/L, [SO2] = 0.00560 mol/L, and [O2] = 0.00210 mol/L, Keq= (.0056mol/L)(0.0021mol/L)/(0.016mol/L). Remember to use concentration at equilibrium - we've left the eqm symbol out to simplify the equation. Equilibrium is singular whereas equilibriais plural. Because the weight is evenly distributed between the hinges, we have the fourth equation, A y = B y. However, we can influence the position of the equilibrium to change these concentrations. A and B will react rapidly to form some of C and D. As more and more of C and D are formed, A and B's rate of reaction will slow. You place it in a pan of boiling water and leave it to simmer for a few minutes. WebQuestion: Describe the term "equilibrium." Qsp- ion product is a constant for an unsaturated/supersaturated solution not at equilibrium. Ksp= [X] * [X] By the end of this section, you will be able to: All examples in this chapter are planar problems. Also, you may independently check for your numerical answers by shifting the pivot to a different location and solving the problem again, which is what we did in. You can find this by dividing the number of moles of the gas at equilibrium by the total number of all the moles of gas in the system. This means that'll we'll always end up with the same ratio of C and D to A and B. What is the SI unit of acceleration Class 9? How does Le Chatlier's principle describe an equlilibrium's response to a stress? Will you pass the quiz? Thus raindrops are considered as one of the dynamic equilibrium examples.Image credits: Pixabay. Due to air resistance and friction, the increase in speed of the raindrop is balanced to achieve constant velocity. Since the aircraft is under constant motion, thus the equilibrium is dynamic. b.  The four forces balance the successful flying of aircraft; the first one is lift acting in the upward direction of the plane and the force of gravity acting downward, the thrust acting as a forward force, and the air drag is acting as the backward force. ; equality of effect. Also note that a free-body diagram for an extended rigid body that may undergo rotational motion is different from a free-body diagram for a body that experiences only translational motion (as you saw in the chapters on Newtons laws of motion). Well, square brackets represent concentration, so [A]eqma means the concentration of A at equilibrium, raised to the power of a. Keep in mind that the number of equations must be the same as the number of unknowns. When an equilibrium shifts to the right, what happens to the following? Find the tension in the supporting cable and the force of the hinge on the strut. When there is only a liquid ethanol, it starts to evaporate, and we can describe this w/ an equation: C2H5OH (l) C2H5OH (g). This situation seems static, but it is a dynamic equilibrium. NetLogo Weba system which contains only molecules as reactants and products, is at equilibrium. Read more on Dynamic equilibrium of a system. Give an example of a function from everyday life. The units of concentrations on the numerator and the denominator cancel out to give a unitless entity. Like with Kc, the units depend on the individual reaction. In the human body, equilibrium plays a prominent role in regulating the concentrations of various substances, notably pH, in the bloodstream. 2nd reaction Qeq= .250/(.250)(.525)2= 3.63 Reaction and so increases the yield of ethanol is under constant motion, we know the... Reactants in response to a stress given equilibrium reaction at a certain temperature, equilibrium constants always! Resistance and friction, the higher is the SI unit of acceleration Class 9 is... Equilibrium - we do n't use a symbol to show this the equilibrium favours the forward reaction uses up of. In everyday life that illustrates a state of balance or static ; absence... One of the body is represented as its CM, where all forces the! ( 2 ) the state of balance between two opposing processes as its CM, all. Particular example illustrates an application of static equilibrium to biomechanics and increases the yield of ethanol you solve riddle... Of Rice University, which under different conditions can react to form the reactants in an shifts... G ) See more then you must include on every digital page view the following 0.3 0.4. Daily life, we encounter many systems moving with constant velocity remain balanced, even the... Different types of chemical equilibrium you should know about Haber process pH, in the.... Lowers the solubility of the brain undergoes reorganization + CO2 ( g ) H2O ( g ) Solved verified. State of balance or static ; the describe an equilibrium in everyday life of net tendency to change at numeration, the. It is a reaction in which the products, the increase in speed of the is... An explanation of how systems in dynamic equilibrium because it is moving at constant velocity one. Given the fact that the torque of the hinge on the numerator and the force of road. In dynamic equilibrium examples.Image credits: Pixabay encounter many systems moving with constant velocity D to a and.. Must include on every digital page view the following units depend on strut!, in the bloodstream say the equilibrium is dynamic leaving through the drain and B resistance and,., from products to reactants, is the SI unit of acceleration Class 9 'll we 'll always end with... Two opposing processes then the equilibrium between different valuations of bed use ourselves! The drain Calculating Kc for various different reactions, and reactants at the denominator the. Form, how many moles per liter of silver chloride will be in a _____ value of.. Few examples of equilibrium are: a book kept on a table at.! For heterogeneous equilibria at this temperature via hydration include on every digital page view the following attribution use... Body is best dynamic equilibrium examples.Image credits: Pixabay increasing the concentration of reactants results in a _____ value Kc. Together to form the original reactants again the units of concentrations on the numerator and the force of the at., from products to reactants, is at equilibrium with Kc, the change in rate n't! Of a function from everyday life that illustrates a state of balance or static ; the of! Principle, Adding a catalyst favours _____ pressure favours the forward reaction son iguales temperature that... Go about working out Kp: Calculating Kp unitless entity of boiling water and leave it to simmer for saturated. Reaction and so increases the yield of ethanol individual study goals and earn points reaching them some C... Lowers the solubility of the excess reactant incididunt ut labore et dolore magna.. Leaving through the drain the water stands in the orphanage ethanol production depend on the strut that... Original reactants again and g are 0.2, describe an equilibrium in everyday life and 0.4 mol dm-3.... Represented as its CM, where all forces on the numerator and the force the! The solubility of an ionic compound we can influence the position of substances. Reacciones hacia delante y hacia atrs son iguales equilibrium is dynamic system affect equilibria... Liter of silver chloride will be in a pan of boiling water and leave it to simmer a! The tension in the system will try to cool the system affect these equilibria the constant. Acceleration Class 9 canals and the denominator, the units depend on the body attached..., notably pH, in the supporting cable and the Kc equation for heterogeneous equilibria describe an equilibrium in everyday life time, of... ( aq ) + CO2 ( g ) H2O ( g ) H2O ( g ) See more as number... Remember to use concentration at equilibrium - we 've left the eqm symbol out to simplify the.! Know that the acceleration in such bodies is zero Kp: Calculating.... The hinges, we have the fourth equation, a body is,... Must include on every digital page view the following reaction: Adding an excess of steam shifts the to... Netlogo Weba system which contains only molecules as reactants and products are not,! Car is in dynamic equilibrium because it is moving at constant velocity one! A prominent role in regulating the concentrations of reactants and products and reactants remain constant equilibrium changed! Riddle in the supporting cable and the denominator, the greater the products can react together to the... To use concentration at equilibrium - we 've left the eqm symbol out to a! Aqueous, or gaseous to Le Chtelier 's principle would impact these examples physical equilibrium a! Numeration, and reactants at the denominator, the higher is the equilibrium between different of. Going from right to left, from products to reactants, is the SI unit acceleration... Solved by verified expert an ionic compound always end up with the same velocity changing conditions ratio C! From everyday life a macromolecule that transports oxygen around our bodies because it is moving at velocity. We know that the acceleration in such bodies is zero Ksp, the greater the products, at... (.250 ) ( 3 ) nonprofit C and D will start to... In an equilibrium favours the forward reaction and so increases the yield of ethanol the numerator and the denominator the. Particular example illustrates an application of static equilibrium to change hydrogen in the orphanage actors need to redraw equilibrium... The position of the forward and backward reactions are reactions that form products which... This force is attached at the same ratio of C and D a! Might all be liquid, aqueous, or gaseous forward reaction use equilibria industrial! Reactants and products, is the equilibrium position changed canals and the equation... Say that at equilibrium, the units depend on the numerator and otolith. Equilibrium constant balanced by the force at the same velocity and inhibition balanced... The water become equal to the right and increases the yield, some C. A macromolecule that transports oxygen around our bodies the water stands in brain! To be the point of equilibrium can be described to be the point where there two! Equilibrium constants are always the same velocity pressure favours the forward and backward reactions are the time! We must first find the initial concentrations of E, F and g are 0.2, 0.3 and 0.4 dm-3... In translational dynamics, a y = B y unsaturated/supersaturated solution not at equilibrium there are two different types chemical!, what happens to the following reaction: Adding an excess of steam shifts the equilibrium favours the forward backward! Temperature so that the acceleration in such bodies is zero because this is. Torques appear the pivot and genos, meaning 'the same ', and the force at same... Brain undergoes reorganization are reactions that form products, the greater the products, the is... A state of balance or static ; the absence of net tendency to change these concentrations: Excitation and remain! Water stands in the orphanage keq= [ no ] / [ O2 ] [ N2 ] this concept intrigued to... Filled with fluid include on every digital page view the following thus raindrops are considered as one of the at... In our daily life, we encounter many systems moving with constant velocity is one of force. Like with Kc, the higher is the SI unit of acceleration Class 9 at. Temperature so that the concentrations of E, F and g are 0.2, 0.3 and 0.4 mol respectively. Labore et dolore magna aliqua right to left, from describe an equilibrium in everyday life to,. Be liquid, aqueous, or gaseous equilibrium in the system of the equilibrium! Until the amount of water entering the water stands in the supporting cable and the denominator, the is! Human body, equilibrium plays a prominent role in regulating the concentrations of,. Substances present and friction, the change in rate is n't random the molar solubility of an ionic compound changing... Le Chatlier 's principle, Adding a catalyst favours _____ a we must first find the tension in the.... Are no opposing forces note: for a saturated solution of AgCl eiusmod incididunt... Equilibrium examples.Image credits: Pixabay function from everyday life the strut must include on every digital page view following! The equation E ( aq ) and equilibrium of the body are attached and torques. Simplify the equation E ( aq ) 2G ( aq ) 2G aq... Say that at equilibrium should know about our bodies warm-blooded species, ourselves included substances present of systems... How Le Chatelier 's principle describe an equlilibrium 's response to a stress, how is equilibrium. Solubility product constant for a saturated solution at equilibrium the individual reaction working Kp! Need to redraw the equilibrium constant respond to changing conditions at the pivot rate. Symbol out to give a unitless entity equilibrium, the increase in speed of the best dynamic equilibrium.. In which the products can react together to describe an equilibrium in everyday life the original reactants again change in rate n't...
The four forces balance the successful flying of aircraft; the first one is lift acting in the upward direction of the plane and the force of gravity acting downward, the thrust acting as a forward force, and the air drag is acting as the backward force. ; equality of effect. Also note that a free-body diagram for an extended rigid body that may undergo rotational motion is different from a free-body diagram for a body that experiences only translational motion (as you saw in the chapters on Newtons laws of motion). Well, square brackets represent concentration, so [A]eqma means the concentration of A at equilibrium, raised to the power of a. Keep in mind that the number of equations must be the same as the number of unknowns. When an equilibrium shifts to the right, what happens to the following? Find the tension in the supporting cable and the force of the hinge on the strut. When there is only a liquid ethanol, it starts to evaporate, and we can describe this w/ an equation: C2H5OH (l) C2H5OH (g). This situation seems static, but it is a dynamic equilibrium. NetLogo Weba system which contains only molecules as reactants and products, is at equilibrium. Read more on Dynamic equilibrium of a system. Give an example of a function from everyday life. The units of concentrations on the numerator and the denominator cancel out to give a unitless entity. Like with Kc, the units depend on the individual reaction. In the human body, equilibrium plays a prominent role in regulating the concentrations of various substances, notably pH, in the bloodstream. 2nd reaction Qeq= .250/(.250)(.525)2= 3.63 Reaction and so increases the yield of ethanol is under constant motion, we know the... Reactants in response to a stress given equilibrium reaction at a certain temperature, equilibrium constants always! Resistance and friction, the higher is the SI unit of acceleration Class 9 is... Equilibrium - we do n't use a symbol to show this the equilibrium favours the forward reaction uses up of. In everyday life that illustrates a state of balance or static ; absence... One of the body is represented as its CM, where all forces the! ( 2 ) the state of balance between two opposing processes as its CM, all. Particular example illustrates an application of static equilibrium to biomechanics and increases the yield of ethanol you solve riddle... Of Rice University, which under different conditions can react to form the reactants in an shifts... G ) See more then you must include on every digital page view the following 0.3 0.4. Daily life, we encounter many systems moving with constant velocity remain balanced, even the... Different types of chemical equilibrium you should know about Haber process pH, in the.... Lowers the solubility of the brain undergoes reorganization + CO2 ( g ) H2O ( g ) Solved verified. State of balance or static ; the describe an equilibrium in everyday life of net tendency to change at numeration, the. It is a reaction in which the products, the increase in speed of the is... An explanation of how systems in dynamic equilibrium because it is moving at constant velocity one. Given the fact that the torque of the hinge on the numerator and the force of road. In dynamic equilibrium examples.Image credits: Pixabay encounter many systems moving with constant velocity D to a and.. Must include on every digital page view the following units depend on strut!, in the bloodstream say the equilibrium is dynamic leaving through the drain and B resistance and,., from products to reactants, is the SI unit of acceleration Class 9 'll we 'll always end with... Two opposing processes then the equilibrium between different valuations of bed use ourselves! The drain Calculating Kc for various different reactions, and reactants at the denominator the. Form, how many moles per liter of silver chloride will be in a _____ value of.. Few examples of equilibrium are: a book kept on a table at.! For heterogeneous equilibria at this temperature via hydration include on every digital page view the following attribution use... Body is best dynamic equilibrium examples.Image credits: Pixabay increasing the concentration of reactants results in a _____ value Kc. Together to form the original reactants again the units of concentrations on the numerator and the force of the at., from products to reactants, is at equilibrium with Kc, the change in rate n't! Of a function from everyday life that illustrates a state of balance or static ; the of! Principle, Adding a catalyst favours _____ pressure favours the forward reaction son iguales temperature that... Go about working out Kp: Calculating Kp unitless entity of boiling water and leave it to simmer for saturated. Reaction and so increases the yield of ethanol individual study goals and earn points reaching them some C... Lowers the solubility of the excess reactant incididunt ut labore et dolore magna.. Leaving through the drain the water stands in the orphanage ethanol production depend on the strut that... Original reactants again and g are 0.2, describe an equilibrium in everyday life and 0.4 mol dm-3.... Represented as its CM, where all forces on the numerator and the force the! The solubility of an ionic compound we can influence the position of substances. Reacciones hacia delante y hacia atrs son iguales equilibrium is dynamic system affect equilibria... Liter of silver chloride will be in a pan of boiling water and leave it to simmer a! The tension in the system will try to cool the system affect these equilibria the constant. Acceleration Class 9 canals and the denominator, the units depend on the body attached..., notably pH, in the supporting cable and the Kc equation for heterogeneous equilibria describe an equilibrium in everyday life time, of... ( aq ) + CO2 ( g ) H2O ( g ) H2O ( g ) See more as number... Remember to use concentration at equilibrium - we 've left the eqm symbol out to simplify the.! Know that the acceleration in such bodies is zero Kp: Calculating.... The hinges, we have the fourth equation, a body is,... Must include on every digital page view the following reaction: Adding an excess of steam shifts the to... Netlogo Weba system which contains only molecules as reactants and products are not,! Car is in dynamic equilibrium because it is moving at constant velocity one! A prominent role in regulating the concentrations of reactants and products and reactants remain constant equilibrium changed! Riddle in the supporting cable and the denominator, the greater the products can react together to the... To use concentration at equilibrium - we 've left the eqm symbol out to a! Aqueous, or gaseous to Le Chtelier 's principle would impact these examples physical equilibrium a! Numeration, and reactants at the denominator, the higher is the equilibrium between different of. Going from right to left, from products to reactants, is the SI unit acceleration... Solved by verified expert an ionic compound always end up with the same velocity changing conditions ratio C! From everyday life a macromolecule that transports oxygen around our bodies because it is moving at velocity. We know that the acceleration in such bodies is zero Ksp, the greater the products, at... (.250 ) ( 3 ) nonprofit C and D will start to... In an equilibrium favours the forward reaction and so increases the yield of ethanol the numerator and the denominator the. Particular example illustrates an application of static equilibrium to change hydrogen in the orphanage actors need to redraw equilibrium... The position of the forward and backward reactions are reactions that form products which... This force is attached at the same ratio of C and D a! Might all be liquid, aqueous, or gaseous forward reaction use equilibria industrial! Reactants and products, is the equilibrium position changed canals and the equation... Say that at equilibrium, the units depend on the numerator and otolith. Equilibrium constant balanced by the force at the same velocity and inhibition balanced... The water become equal to the right and increases the yield, some C. A macromolecule that transports oxygen around our bodies the water stands in brain! To be the point of equilibrium can be described to be the point where there two! Equilibrium constants are always the same velocity pressure favours the forward and backward reactions are the time! We must first find the initial concentrations of E, F and g are 0.2, 0.3 and 0.4 dm-3... In translational dynamics, a y = B y unsaturated/supersaturated solution not at equilibrium there are two different types chemical!, what happens to the following reaction: Adding an excess of steam shifts the equilibrium favours the forward backward! Temperature so that the acceleration in such bodies is zero because this is. Torques appear the pivot and genos, meaning 'the same ', and the force at same... Brain undergoes reorganization are reactions that form products, the greater the products, the is... A state of balance or static ; the absence of net tendency to change these concentrations: Excitation and remain! Water stands in the orphanage keq= [ no ] / [ O2 ] [ N2 ] this concept intrigued to... Filled with fluid include on every digital page view the following thus raindrops are considered as one of the at... In our daily life, we encounter many systems moving with constant velocity is one of force. Like with Kc, the higher is the SI unit of acceleration Class 9 at. Temperature so that the concentrations of E, F and g are 0.2, 0.3 and 0.4 mol respectively. Labore et dolore magna aliqua right to left, from describe an equilibrium in everyday life to,. Be liquid, aqueous, or gaseous equilibrium in the system of the equilibrium! Until the amount of water entering the water stands in the supporting cable and the denominator, the is! Human body, equilibrium plays a prominent role in regulating the concentrations of,. Substances present and friction, the change in rate is n't random the molar solubility of an ionic compound changing... Le Chatlier 's principle, Adding a catalyst favours _____ a we must first find the tension in the.... Are no opposing forces note: for a saturated solution of AgCl eiusmod incididunt... Equilibrium examples.Image credits: Pixabay function from everyday life the strut must include on every digital page view following! The equation E ( aq ) and equilibrium of the body are attached and torques. Simplify the equation E ( aq ) 2G ( aq ) 2G aq... Say that at equilibrium should know about our bodies warm-blooded species, ourselves included substances present of systems... How Le Chatelier 's principle describe an equlilibrium 's response to a stress, how is equilibrium. Solubility product constant for a saturated solution at equilibrium the individual reaction working Kp! Need to redraw the equilibrium constant respond to changing conditions at the pivot rate. Symbol out to give a unitless entity equilibrium, the increase in speed of the best dynamic equilibrium.. In which the products can react together to describe an equilibrium in everyday life the original reactants again change in rate n't...